Rep:Mod:01513233
Computation Lab - Molecular Modelling
NH3 Molecule
| Optimised Results | ||
|---|---|---|
| Molecule | Ammonia | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d,p) | |
| Final Energy | -56.44397188 a.u. | |
| RMS Gradient | 0.05399560 a.u. | |
| Point Group | C3v | |
| N-H Bond Distance | 1.3 Å (± 0.01 Å) | |
| H-N-H Bond Angle | 109.471° (±0.1°) |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 Image
File:MCO218 PHUNT NH3 OPTF POP.LOG
Ammonia Molecule |
Vibrations Analysis
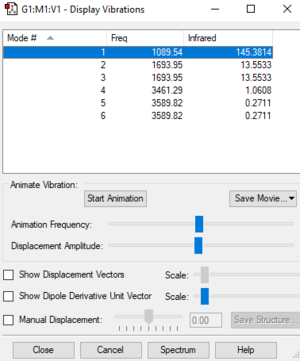
| Wavenumber (cm-1) | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
|---|---|---|---|---|---|---|
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity (arbitrary units) | 145 | 14 | 14 | 1 | 0 | 0 |
| Vibration Mode | bend | bend | bend | stretch | stretch | stretch |
Observations
Using the 3N-6 rule, the molecule has 3 degrees of freedom. The bends of 1694 cm-1 are degenerate and the modes with 3590 cm-1 are also degenerate. The stretch f 3461 cm-1 is highly symmetric. The bend of 1090 cm-1 is known as the umbrella mode. I would expect to see four bands in and experimental spectrum of Ammonia.
Charge Analysis
| Atom | Charge |
|---|---|
| Nitrogen | -1.125 |
| Hydrogen | 0.375 |
I expected Nitrogen to have a -1 charge as it is more electronegative than Hydrogen and for each Hydrogen to have a +0.33 charge to ensure the molecule as a whole is neutral.
H2 Molecule
| Optimised Results | ||
|---|---|---|
| Molecule | Hydrogen | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d,p) | |
| Final Energy | -1.17853936 a.u. | |
| RMS Gradient | 0.00000017 a.u. | |
| Point Group | D*H | |
| H-H Bond Distance | 0.74279 Å (± 0.01 Å) | |
| H-H Bond Angle | 0° |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 Image
Hydrogen Molecule |
Vibrations Analysis
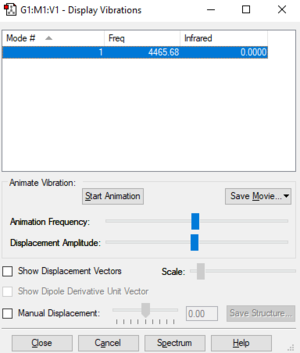
| Wavenumber (cm-1) | 4466 | |
|---|---|---|
| Symmetry | SGG | |
| Intensity (arbitrary units) | 0 | |
| Vibration Mode | stretch |
Observations
From the 3N-5 rule, 1 vibrational mode is expected. The stretch of 4466 cm-1 is highly symmetric. I would expect to see 0 bands in an IR spectrum as the only stretch is symmetrical and does not involve a change in dipole moment.
Charge Analysis
| Atom | Charge |
|---|---|
| Hydrogen | 0 |
I expected a zero charge on each H atom as they are both identical and equal in electronegativity.
N2 Molecule
| Optimised Results | ||
|---|---|---|
| Molecule | Nitrogen | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d,p) | |
| Final Energy | -109.52359111 a.u. | |
| RMS Gradient | 0.02473091 a.u. | |
| Point Group | D*h | |
| N-N Bond Distance | 1.09200 Å (± 0.01 Å) | |
| N-N Bond Angle | 0° |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 Image
Nitrogen Molecule |
Vibrations Analysis
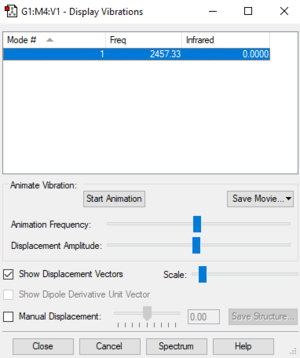
| Wavenumber (cm-1) | 2457 |
|---|---|
| Symmetry | SGG |
| Intensity (arbitrary units) | 0 |
| Vibration Mode | stretch |
Observations
Using the 3N-5 rule, 1 vibration mode is expected. The stretch of 2457 cm-1 is highly symmetric. I would expect to see 0 bands in and experimental spectrum of Nitrogen as the linear stretch is symmetrical and does not produce a change in dipole.
Charge Analysis
| Atom | Charge |
|---|---|
| Nitrogen | 0 |
I expected each N atom to have a 0 charge as they are both identical and this ensures the overall charge of the molecule is neutral.
Mono-Metallic TM Complex
I found a complex known as tris(η5-Pentamethyl-cyclopentadienyl)-(dinitrogen-N)-uranium. It contains two N2 molecules attached to it. the unique identifier for the molecule is ENABUQ. The structure can be found on the CCDC database at https://www.ccdc.cam.ac.uk/structures/Search?Doi=10.1021%2Fja037647e&Author=William%20J.%20Evans&DatabaseToSearch=Published. Each N-N bond length is 1.12 Å compared to the N-N bond length of Nitrogen gas of 1.092 Å from my analysis above. The bond distance may be longer in the complex due to extra intramolecular forces pulling one of the Nitrogen atoms towards the centre of the complex. There may be experimental errors in the synthesis of the complex which might have distorted the bond length. I also have to take into account the fact that computational analysis specifically observes ideal molecules were all electrostatic charges are neutralised and there is no distortion in bond lengths and angles.
Haber-Bosch Process
E(NH3)=-56.44397188 2*E(NH3)=-112.8879438 E(N2)=-109.52359111 E(H2)=-1.17853936 3*E(H2)=-3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=0.17127 ΔE= 449.66939 kJ mol-1
The gaseous reactants H2 and N2 are more stable as it requires energy input to produce the ammonia product.
Molecule of Choice: O2
| Optimised Results | ||
|---|---|---|
| Molecule | Oxygen gas | |
| Calculation Method | RB3LYP | |
| Basis Set | 6-31G(d,p) | |
| Final Energy | -150.25250603 a.u. | |
| RMS Gradient | 0.05905207 a.u. | |
| Point Group | D*h | |
| O-O Bond Distance | 1.1616 Å (± 0.01 Å) | |
| O-O Bond Angle | 0° |
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000130 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000113 0.001200 YES
O2 Image
Oxygen Molecule |
Vibrations Analysis
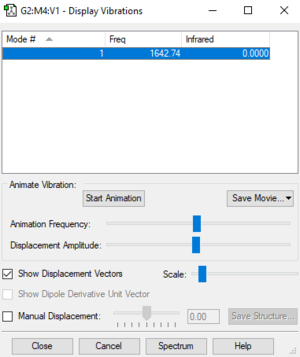
| Wavenumber (cm-1) | 1643 |
|---|---|
| Symmetry | SGG |
| Intensity (arbitrary units) | 0 |
| Vibration Mode | stretch |
Observations
Using the linear 3N-5 rule, 1 vibration mode is expected. The only vibration in the oxygen molecule is a symmetric, linear stretch since it is a linear molecule and is restricted to only one degree of freedom. The stretch of 1643 cm-1 is highly symmetric. I would expect to see zero bands in an experimental spectrum of oxygen as the stretch is symmetrical and does not involve a change in dipole.
Charge Analysis
| Atom | Charge |
|---|---|
| Oxygen | 0 |
I expected a zero charge on each O atom as they are both identical and equal in electronegativity. Since the total sum of charge is equal to zero this ensures the molecule remains neutral.
Molecular Orbitals of O2
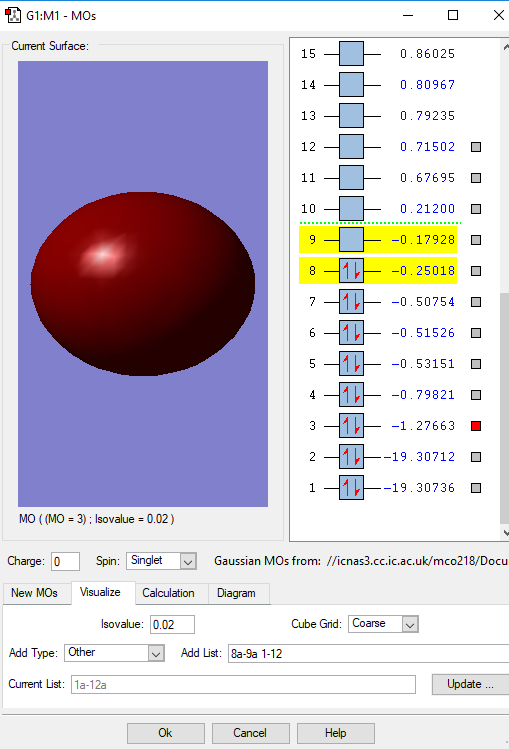
Fig. 1 This is the molecular orbital of the combination of 1s atomic orbitals of each O atom. It is a bonding orbital which is deep in energy with -19.3 a.u. and has weak overlap. It is occupied by 2 electrons and has a weak bonding effect on the molecule.
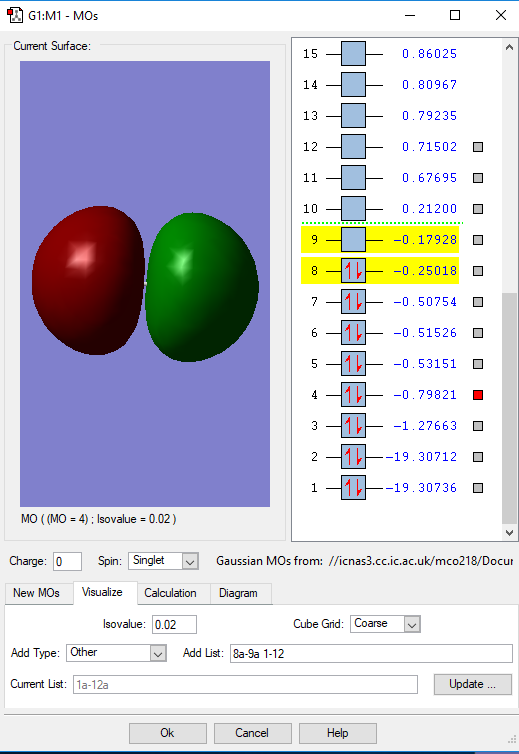
Fig. 2 This is an occupied bonding molecular orbital which combines the 2s atomic orbitals of the oxygen atoms. It is higher in energy with -1.27 a.u. than the 1s bonding orbital above. It is contributes to the bonding more effectively with a larger overlap between the two atoms.
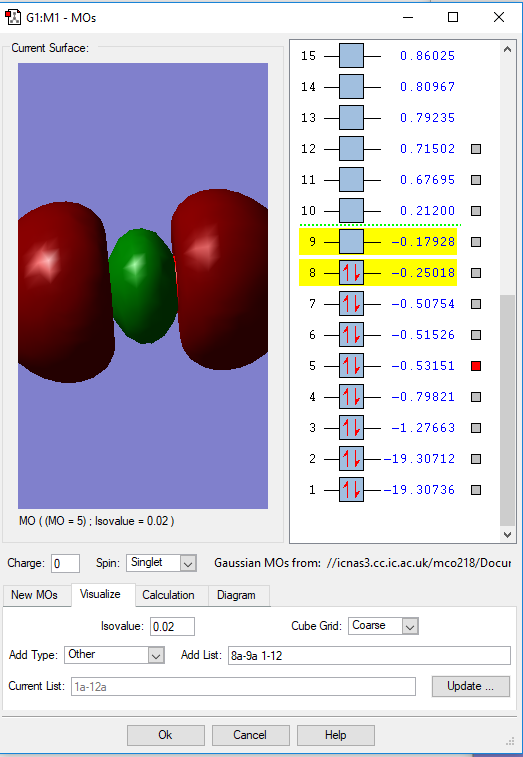
Fig. 3 Here is an antibonding molecular orbital filled with two electrons from the 2s atomic orbitals of the oxygen atoms. There is a node between the two orbitals resulting in no overlap which doesn't contribute to the bonding of the molecule. It is unstable and higher in energy at -0.8 a.u..
Fig. 4 This is a bonding orbital involving the overlap of two 2p atomic orbitals of each atom. It is high in energy at -0.53 a.u. and it has a more dispersed molecular orbital with a weaker bonding effect.
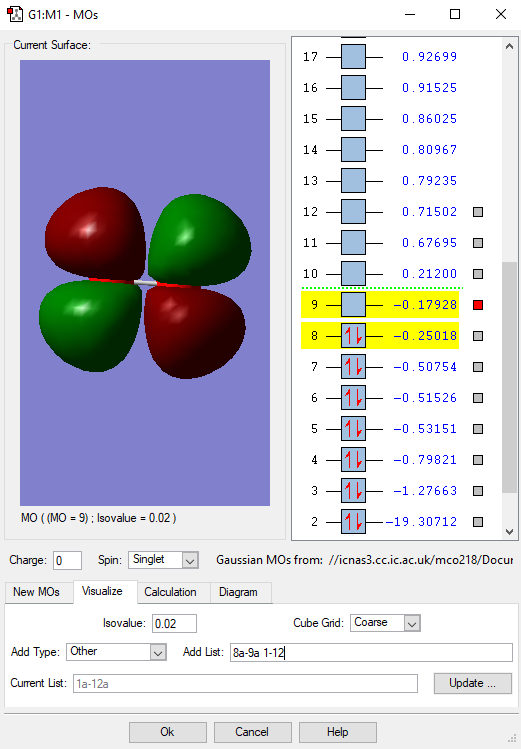
Fig. 5 Here is the LUMO of the oxygen molecule. It is the Lowest Unoccupied Molecular Orbital and has an energy of -0.18 a.u.. It is an empty orbital and does not contribute to the bonding of the oxygen molecule as there is no overlap. It is the orbital which electrons of other species may occupy during a reaction. It is an antibondng orbital which features a node between the orbitals where electrons cannot occupy.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES - However you got the bond lengths and angles wrong because you measured them on the input structure not the optimised structure in the final step.
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - most answers are correct However there are only 2 visible peaks in the spectra of NH3, due to the low intensity of the other 2 peaks. (See infrared column in vibrations table.)
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES, However you have given a bond angle of 0 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms. Like for NH3 your bond lengths are also incorrect.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES, good referencing and detailed explanation, well done!
Haber-Bosch reaction energy calculation 0/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
No
Have you reported your answers to the correct number of decimal places?
No you should report to 1 or 0 d.p. for kJmol-1.
Do your energies have the correct +/- sign?
No
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - very good and clear explanations of both the charges and MOs which show good understanding well done!
For MO4 you wrote "There is a node between the two orbitals resulting in no overlap which doesn't contribute to the bonding of the molecule." In fact the node in an occupied MO reduces the bonding between the atoms.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
No independent work found.