Rep:Mod:01500798
NH3
Molecule Information
Molecular name: Ammonia
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy:-56.55776873 au
Point Group: C3V
N-H bond distance:1.02Å
H-N-H bond angle:37°
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol of NH3
Ammonia |
link
File:QUANWENT NH3 OPTF POP.LOG
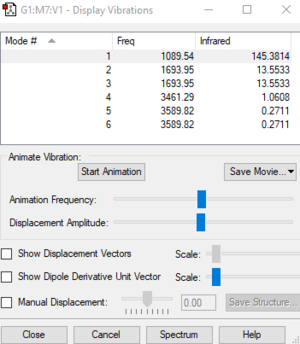
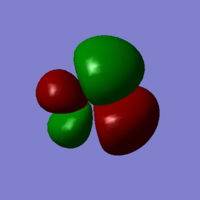
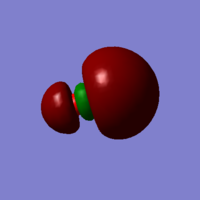
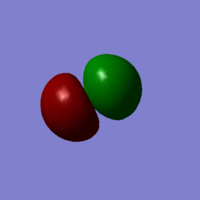
NH3 Vibrations
| Wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| Image | 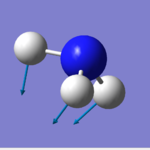 |
 |
 |
 |
 |
|
Answer of Questions
how many modes do you expect from the 3N-6 rule?
- 3 x 4 - 6 = 6 modes
which modes are degenerate (ie have the same energy)?
- Modes with wavenumbers of 1694 cm^-1 and 3590 cm^-1.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
- Modes with the wavenumber of 1090 cm^-1 and 1694 cm^-1 are "bending" vibrations,modes with the wavenumber of 3461 cm^-1 and 3590 cm^-1 are "bond stretch" vibrations.
which mode is highly symmetric?
- Mode with the wavenumber of 3461 cm^-1.
one mode is known as the "umbrella" mode, which one is this?
- Mode with the wavenumber of 1090 cm^-1.
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
- Two bands. Because degenerate modes show one band.And the mode with the wavenumber of 3461 cm^-1 has intensity 1.
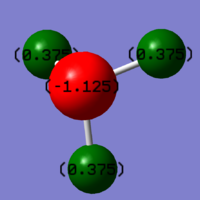
Charge Analysis
In expectation, N is more electronegative than H and should have negative charge,H should have positive charge. The results show that the charge is -1.125 on N and 0.375 on H.
N2
Molecule Information
Molecular name: Nitrogen
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -109.52412868 au
Point Group: D*H
N-N bond distance:1.11Å
N-N bond angle:180°
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000001 0.001800 YES RMS Displacement 0.000002 0.001200 YES
Jmol of N2
N2 |
Link
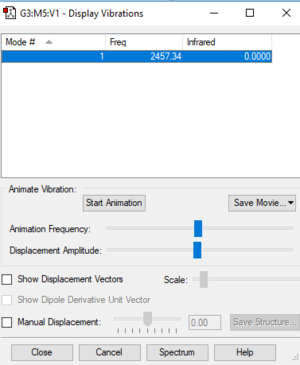
N2 Vibrations
Vibration mode of N2
| Wavenumber cm^-1 | 2457 |
| Symmetry | SGG |
| Intensity Arbitrary Units | 0 |
| Image | 
|
Charge Analysis
In expectation, the charge on both N should be zero since there is no resultant dipole moment. The results show that the charge is on both N is 0.
H2
Molecule Information
Molecular name: Hydrogen
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -1.17853936 au
Point Group: D*H
H-H bond distance:0.74Å
H-H bond angle:180°
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Jmol of H2
H2 |
Link
H2 Vibrations
Vibration mode of H2
| Wavenumber cm^-1 | 4466 |
| Symmetry | SGG |
| Intensity Arbitrary Units | 0 |
| Image | 
|
Charge Analysis
In expectation, the charge on both H should be zero since there is no resultant dipole moment. The results show that the charge is on both H is 0.
Structure and Reactivity
mono-metallic TM complex DEKFUX
Link to the complex:[1]
There is one N-N triple bond in the complex. The bong length of this triple bod is 1.086. The N-N triple bond length in Nitrogen gas is 1.10550 which is slightly longer. I think the reason that causes the difference in bond length is that the bond length calculated for Nitrogen gas is under perfect condition whereas there are other factors influencing the value in reality. The transition metal attached in crystal structure help to stabilize the N-N triple bond. So the bong length in crystal structure is shorter.
Energy for Reaction
E(NH3)= -56.557769 au
2*E(NH3)= -113.115538 au
E(N2)= -109.5241287 au
E(H2)= -1.1785394 au
3*E(H2)= -3.5356180 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 au = -146.8 kJ/mol
The ammonia product is more stable since the change in energy is negative.
CO2
Molecule Information
Molecular name: Carbon dioxide
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -188.58093945 au
Point Group: D*H
C-O bond distance:1.17Å
C-O bond angle:180°
Item Table
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000015 0.001200 YES
Jmol of CO2
CO2 |
Link
CO2 Vibrations
Vibration mode of CO2
| Wavenumber cm-1 | 640 | 640 | 1372 | 2436 |
| Symmetry | PIU | PIU | SGG | SGU |
| Intensity arbitrary units | 31 | 31 | 0 | 546 |
| Image |  |
 |
 |
|
Answer of Questions
how many modes do you expect from the 3N-5 rule?
- 3 x 4 - 6 = 4 modes
which modes are degenerate (ie have the same energy)?
- Modes with wavenumbers of 640 cm^-1.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
- Modes with the wavenumber of 640 cm^-1 are "bending" vibrations,modes with the wavenumber of 1372 cm^-1 and 2436 cm^-1 are "bond stretch" vibrations.
which mode is highly symmetric?
- Mode with the wavenumber of 1372 cm^-1.
how many bands would you expect to see?
- Two bands. Because degenerate modes show one band.And the mode with the wavenumber of 1372 cm^-1 has intensity 0.
Charge Analysis
Charge on C: 1.022
Charge on O: -0.511
In expectation, the charge on O should be negative since O is more electronegative than C.
Molecular Orbitals
Molecular orbital 12
-AOs contribute to the MO: 2p(C) 2p(O) 2p(O)
-Antibonding or bonding: Anitibonding
-Energy: 0.02992 au (LUMO)
-Occupied or unoccupied: Unoccupied
Molecular orbital 11
-AOs contribute to the MO: 2p(O) 2p(O)
-Antibonding or bonding: Antibonding
-Energy: -0.36997 au (HOMO)
-Occupied or unoccupied: Occupied
Molecular orbital 8
-AOs contribute to the MO: 2p(C) 2p(O) 2p(O)
-Antibonding or bonding: Bonding
-Energy: -0.51277 au
-Occupied or unoccupied: Occupied
Molecular orbital 4
-AOs contribute to the MO: 2s(O) 2s(O)
-Antibonding or bonding: Bonding
-Energy: -1.16099 au (deep in energy)
-Occupied or unoccupied: Occupied
Molecular orbital 7
-AOs contribute to the MO: 2p(C) 2p(O) 2p(O)
-Antibonding or bonding: Antibonding
-Energy: -0.51655 au
-Occupied or unoccupied: Occupied
Extra: CO
Molecule Information
Molecular name: Carbon monoxide
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -113.30945314 au
Point Group: C*V
C-O bond distance:1.14Å
C-O bond angle:180°
Item Table
Item Value Threshold Converged? Maximum Force 0.000032 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Jmol of CO
CO |
Link
CO Vibrations
Vibration mode of CO
| Wavenumber cm-1 | 2209 |
| Symmetry | SG |
| Intensity arbitrary units | 68 |
| Image | 
|
Charge Analysis
Charge on C: 0.506
Charge on O: -0.506
In expectation, the charge on O should be negative since O is more electronegative than C.
Molecular Orbitals
Molecular orbital 8
-AOs contribute to the MO: 2p(C) 2p(O)
-Antibonding or bonding: Anitibonding
-Energy: -0.02177 au (LUMO)
-Occupied or unoccupied: Unoccupied
Molecular orbital 7
-AOs contribute to the MO: 2s(C) 2p(O)
-Antibonding or bonding: Bonding
-Energy: -0.37145 au (HOMO)
-Occupied or unoccupied: Occupied
Molecular orbital 6
-AOs contribute to the MO: 2p(C) 2p(O)
-Antibonding or bonding: Bonding
-Energy: -0.46743 au
-Occupied or unoccupied: Occupied
Molecular orbital 4
-AOs contribute to the MO: 2s(C) 2s(O)
-Antibonding or bonding: Antibonding
-Energy: -0.57004 au
-Occupied or unoccupied: Occupied
Molecular orbital 3
-AOs contribute to the MO: 2s(C) 2s(O)
-Antibonding or bonding: Bonding
-Energy: -1.15791 au
-Occupied or unoccupied: Occupied
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES - but you have given a H-H-N angle instead of a H-N-H bond angle. 37 degrees would be an extremely usual bond angle.
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES. However you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES - However you wrote "The bong length of this triple bod" - you should take a lot more care over your spelling as it is hard to communicate complicated concepts if your use of english is not clear for the reader.
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - correct answers overall well done! You could have explained the MOs in more detail.
Independence 1/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
Do an extra calculation on another small molecule, or
You did an extra calculation well done!
Do some deeper analysis on your results so far