Rep:Mod:01413765
NH3
Optimization of NH3
Molecule name: NH3
Calculation method: RB3LYP
Basis set: 6-31G(D,P)
Final energy E(RB3LYP): -56.55776873 a.u.
Point group: C3v
Optimised N-H bond distance: 1.01798 Å
Optimised H-N-H bond angle: 105.741°
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 |
The optimisation file is liked to here
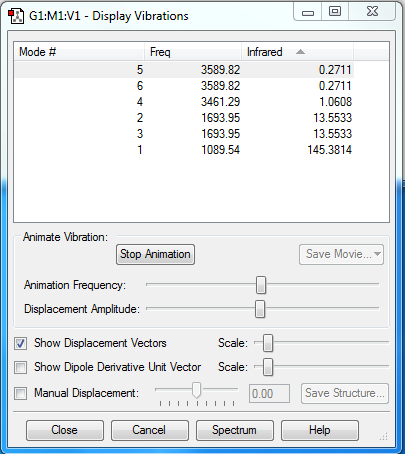
Vibrations of NH3
In theory, the number of the vibration modes of a non-linear molecule is expected to be 3N-6. In terms of the molecule NH3, it is supposed to be 6.
From the experimental result shown above, there are indeed 6 modes, among which Mode 5 and Mode 6 are degenerate with a frequency 3589.82 and Mode 2 and 3 are degenerate with a frequency of 1693.95.
Bending vibrations: The first three (5, 6,4) Stretching vibrations: The last three (2,3,1)
The highly symmetric mode: 4 "Umbrella" mode: 1
In theory, the number of bands expected to be seen in an experimental spectrum of gaseous ammonia is 4; However, in practical there may be only two bands observed since the intensities corresponding to the frequency 3589.82 and 3461.29 are 0.2711 and 1.0608 respectively, which are relatively small because of little dipole change of these vibration modes. And there may be instrumental errors and noise present in the measurement.
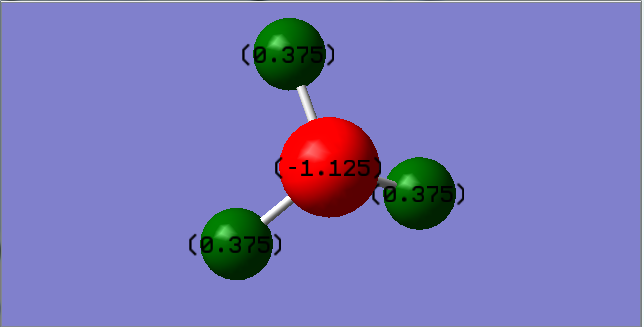
Charge distribution of NH3
The charge of N and H in ammonia is expected to be -3 and +1 respectively according to the common oxidation states of the two elements and their relative electronegativity.
However, the experimentally calculated value is -1.125 and +0.375 for N and H respectively
N2
Optimisation of N2
Molecule name: N2
Calculation method: RB3LYP
Basis set: 6-31G(D,P)
Final energy E(RB3LYP): -109.52412868 a.u.
Point group: Dinfv
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is liked to here
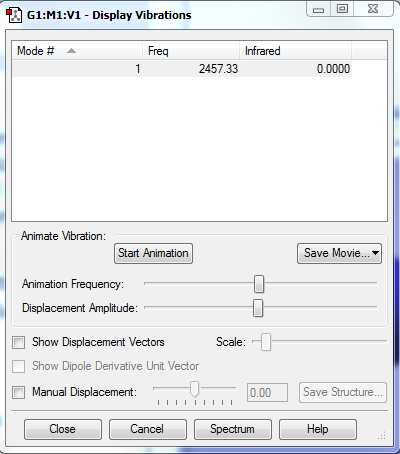
No band is expected to be observed in IR, since there is no dipole change in the vibration of N2
H2
Optimisation of H2
Molecule name: H2
Calculation method: RB3LYP
Basis set: 6-31G(D,P)
Final energy E(RB3LYP): -1.17853936 a.u.
Point group: Dinfv
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
The optimisation file is liked to here
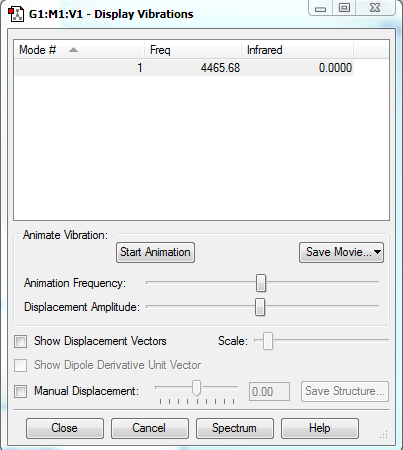
No band is expected to be observed in IR, since there is no dipole change in the vibration of H2
Reaction energy of N2 + 3H2+ -> 2NH3
E(NH3)= -56.5577687 a.u.
2*E(NH3)= -113.1155375 a.u.
E(N2)= -109.5241287 a.u.
E(H2)= -1.1785394 a.u.
3*E(H2)= -3.5356181 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.= -146.82 kJ/mol
Since ΔE is negative, the ammonia product is more stable than the gaseous reactants.
[NH4]+
Optimization of [NH4]+
Molecule name: [NH4]+
Calculation method: RB3LYP
Basis set: 6-31G(D,P)
Final energy E(RB3LYP): -56.90586368 a.u.
Point group: Td
Optimised bond length: 1.02678 Å
Optimised bond angle: 109.471°
Item Value Threshold Converged? Maximum Force 0.000449 0.000450 YES RMS Force 0.000240 0.000300 YES Maximum Displacement 0.001034 0.001800 YES RMS Displacement 0.000553 0.001200 YES
NH4 |
The optimisation file is liked to here
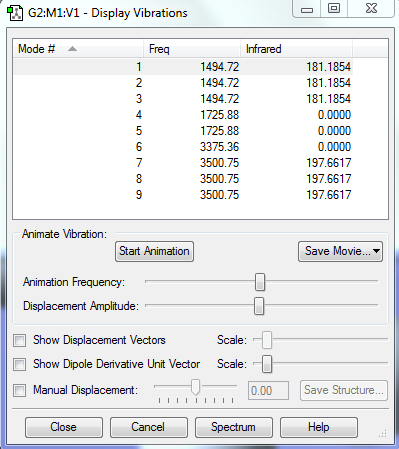
Vibrations of [NH4]+
In theory, the number of the vibration modes of the molecule [NH4]+, it is supposed to be 9
From the experimental result shown above, there are indeed 9 modes, among which Mode 1,2,3 are degenerate with a frequency 1494.72; Mode 4,5 are degenerate with a frequency of 1725.88; Mode 7,8,9 are degenerate with a frequency of 3500.75
Bending vibrations: The first five Stretching vibrations: The last four
The number of bands expected to be seen in an experimental spectrum of gaseous ammonia: 2
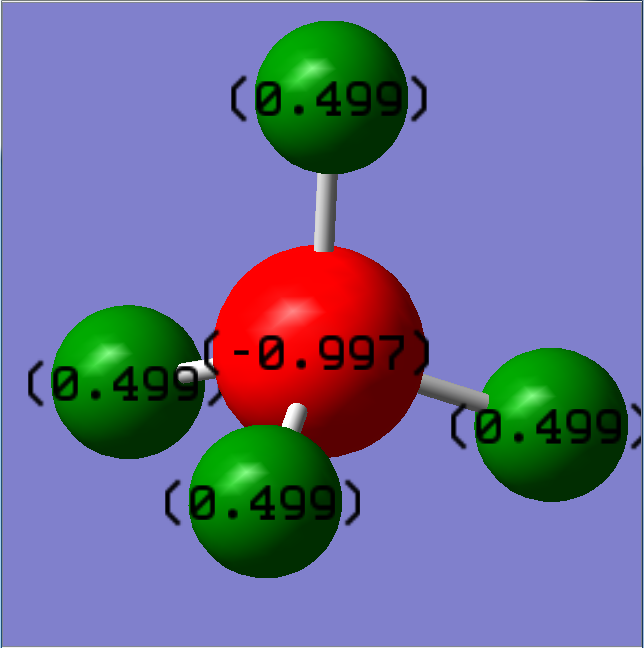
Charge distribution of [NH4]+
The charge of N and H in ammonium ion is expected to be -3 and +1 respectively according to the common oxidation states of the two elements and their relative electronegativity.
However, the experimentally calculated value is -0.997 and +0.499 for N and H respectively
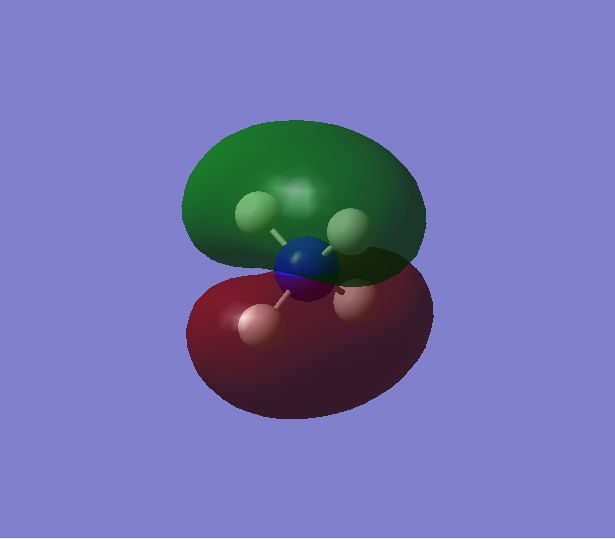
Analysis of molecular orbitals of [NH4]+
Contribution from AOs: 1s(N)
Type of bonding: Non-bonding
Energy: -14.71515 a.u. (deep in energy)
Occupied with two electrons, but does not contribute to bonding
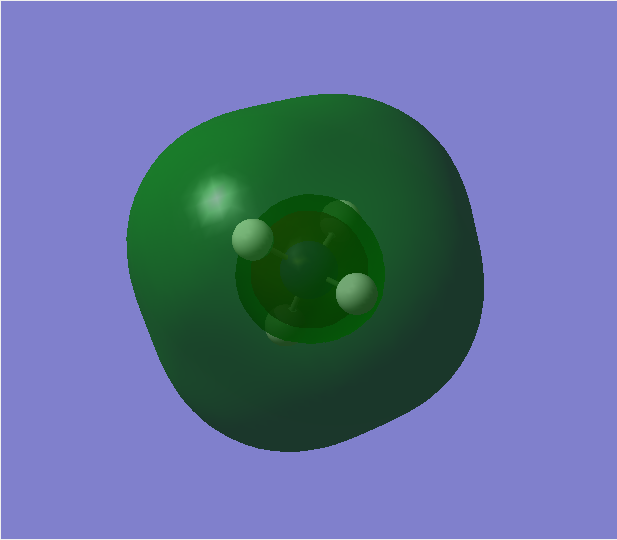
Contribution from AOs: 2s(N) and 1s(H)
Type of bonding: Bonding (contributes to bonding)
Energy: -1.24860 a.u.(deep in energy)
Occupied with two electrons, contributes to bonding
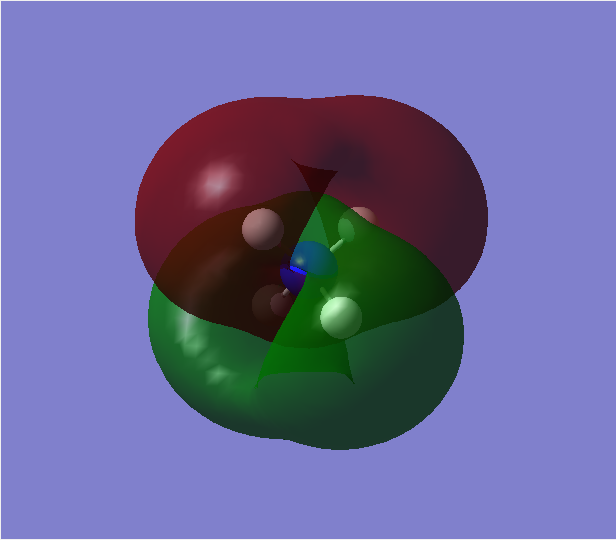
Contribution from AOs: 2p(N) and 1s(H)
Type of bonding: Bonding
Energy: -0.82511 a.u. (HOMO)
Occupied with two electrons, contributes to bonding
Contribution from AOs: 2s(N) and 1s(H)
Type of bonding: Anti-bonding
Energy: -0.20981 a.u. (LUMO)
Unoccupied, does not contribute to bonding
Contribution from AOs: 2p(N) and 1s(H)
Type of bonding: Anti-bonding
Energy: -0.12816 a.u. (High in energy)
Unoccupied, does not contribute to bonding