Rep:Mod:01389684
NH3 Molecule
test molecule |
Summary Information
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy(au) | -56.55776873 |
| RMS Gradient | 0.00000485 |
| Point Group | C3V |
| N-H Bond Length(Angstroms) | 1.01798 |
| H-N-H Bond Angle | 105.741 |
Item table for optimised NH3 Molecule
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
The optimisation file is linked to NH3 Molecule
NH3 Vibrations
From the 3N-6 rule you would expect 6 modes of vibrations for NH3. Modes 2 and 3 are degenerate as well as 5 and 6. Modes 1,2 and 3 are bending vibrations and modes 4,5 and 6 are bond stretching vibrations. Mode 4 is highly symmetric. Mode 1 is the umbrella mode due to the movement of its bending vibrations making it look like an umbrella. 2 bands would be visible in an experimental spectrum of gaseous ammonia despite there being 4 different energy modes as the change in dipole moment in the stretching frequencies (modes 4, 5 and 6) are so small that the intensities of the peaks are very small and so would not be visible in an experimental spectrum especially with noise present.
Charges in the NH3 Molecule
N= -1.125 H= 0.375 This is expected as Nitrogen is more electronegative than Hydrogen and so a more negative charge for Nitrogen would be expected.
N2 Molecule
test molecule |
Summary Information
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy(au) | -109.524 |
| RMS Gradient | 0.00000060 |
| Point Group | D*H |
| N-N Bond Length(Angstroms) | 1.10550 |
Item table for optimised N2 Molecule
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
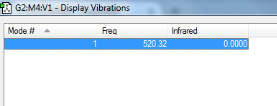
N2 Vibrations
This table shows that Nitrogen would not be infrared active as there is no change in permanent dipole moment.
N2 Molecular Orbitals
H2 Molecule
test molecule |
Summary Information
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy(au) | -1.179 |
| RMS Gradient | 0.00000017 |
| Point Group | D*H |
| H-H Bond Length(Angstroms) | 0.74279 |
Item table for optimised H2 Molecule
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 Vibrations
This table shows that Hydrogen will not be infrared active as there is no change in permanent dipole moment.
Energy value for Haber-Bosch Process
| E(NH3) | -56.55776873au |
| 2*E(NH3) | -113.11553750au |
| E(N2) | -109.52412868au |
| E(H2) | -1.17853936au |
| 3*E(H2) | -3.53561808au |
| ΔE | -0.05579070au |
| ΔE | -146.48KJ/mol (2dp) |
The ammonia product is more stable due to it being an exothermic reaction as the ΔE value is -146.48 KJ/mol (2dp) and so the product (ammonia) will have a lower energy than the reactants, making it more stable.
Cl2 Molecule
test molecule |
Summary Information
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy(au) | -920.350 |
| RMS Gradient | 0.00002510 |
| Point Group | D*H |
| Cl-Cl Bond Length(Angstroms) | 2.04174 |
Item Table for optimised Cl2 Molecule
Item Value Threshold Converged? Maximum Force 0.000043 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000121 0.001800 YES RMS Displacement 0.000172 0.001200 YES
Cl2 Vibrations
This table shows that Cl2 will not be infrared active as there is no change in permanent dipole moment.
Charges on Cl2 Molecule
There are no charges on the chlorine atoms as each atom has the same electronegativity meaning there will be no dipole moment.