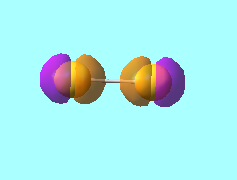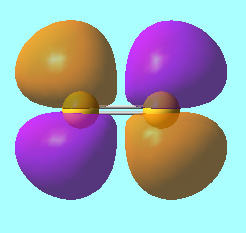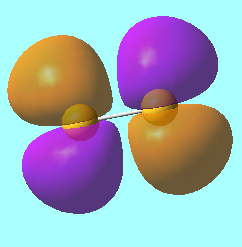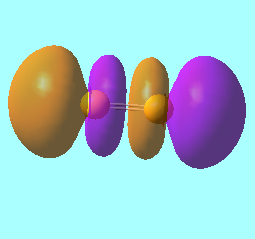Rep:Mod:01363194hbell
NH3 Molecule
Optimising NH3
Ammonia Molecule |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -56.55776873 a.u. |
| RMS Gradient Norm | 0.00000485 a.u. |
| Point Group | C3V |
N-H Bond Length: 1.01798Å
H-N-H Bond Angle: 105.741°
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
The optimisation file is liked to here
Vibrational Analyis
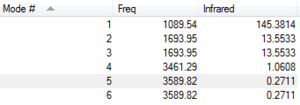
From the 3N-6 rule for NH3, you would expect to see six modes of vibration. This is because NH3 has four atoms, and (3x4)-6 = 6. There are two sets of degenerate modes. Firstly, modes 2 and 3 are degenerate as they both have a frequency of 1693.95cm-1. Also, modes 5 and 6 are degenerate since they both have frequencies of 3589.82cm-1. Modes 1,2 and 3 are "bending" vibrations and modes 4,5 and 6 are "bond stretch" vibrations. Mode 4 is a highly symmetric mode. The mode known as the "umbrella" mode is mode 1. On an experimental spectrum of gaseous ammonia you would expect to see four bands; one corresponding to each different frequency of vibration.
Charge Analysis
The charge on the nitrogen atom is -1.125D and the charge on each of the hydrogen atoms is 0.375D. You would expect nitrogen to be negative and the hydrogens to be positive because nitrogen is more electronegative than hydrogen so there is an uneven distribution of electrons within the bonds giving polar bonds. Since NH3 is trigonal pyramidal the polarity within the molecule is not cancelled, giving the molecule an overall dipole with nitrogen being negative.
N2 Molecule
Nitrogen Molecule |
Optimising N2
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -109.52412868 a.u. |
| RMS Gradient Norm | 0.00000060 a.u. |
| Point Group | D*H |
N-N Bond Length: 1.10550Å
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is liked to here
Vibrational Analysis

From the table to the right it can be seen that there is only one mode of vibration for the N2 molecule. This is because the molecule is a simple diatomic so it has 3N-5 vibrational modes, where N=2, so (3x2)-5= 1 vibrational mode. This mode of vibration is a simple stretch. It can be seen by the fact that the IR column shows a value of 0 that the N2 molecule is not IR active; this is because there is no change in dipole when the bond stretches.
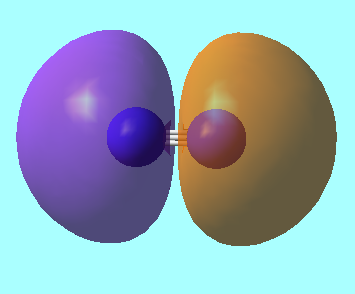
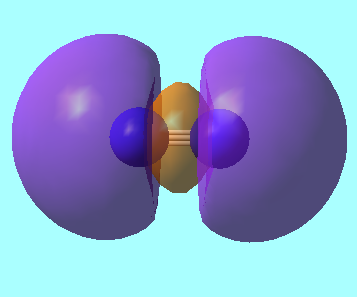
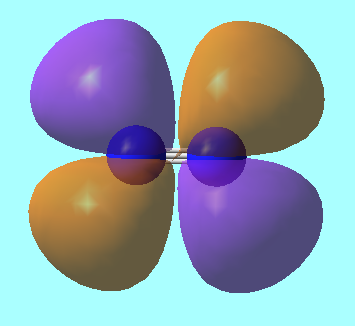
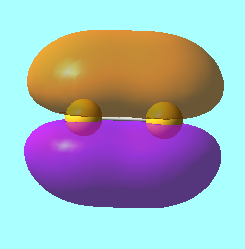
N2 Molecular Orbitals
MO 3: The 2s bonding molecular orbital of energy, E = -1.12383 a.u.
MO 4: The 2s antibonding molecular orbital of energy, E = -0.55342 a.u.
MO 5: The 2p π molecular orbital of energy, E = -0.46240 a.u.
MO 7: The HOMO, a σ bonding orbital from overlapping 2p orbitals of energy, E = -0.46688 a.u.
MO 8: The LUMO, from antibonding contributions of perpendicular 2p orbitals. It has energy, E = -0.02412 a.u.
H2 Molecule
Hydrogen Molecule |
Optimising H2
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -1.17853936 a.u. |
| RMS Gradient Norm | 0.00000017 a.u. |
| Point Group | D*H |
H-H Bond Length: 0.74279Å
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
The optimisation file is liked to here
Vibrational Analysis

The table to the right shows one mode of vibration. Like nitrogen, hydrogen is a simple diatomic so the number of modes of vibration is (3x2)-5=1. The mode of vibration corresponds to a stretch, and as there is no change of dipole moment in this stretch it is not IR active. The frequency of the H2 vibrational mode is higher than that of N2. Even though the N2 triple bond is much stronger than the H2 single bond, the hydrogen molecule's reduced mass is much lower than that of the nitrogen molecule, outweighing the difference in bond strength.
Finding the energy change of the Haber-Bosch reaction
The equation for the Haber-Bosch reaction is as follows: N2(g) + 3H2(g) → 2NH3(g)
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.
ΔE in kJmol-1 = -0.0557907 x 2625.5 = -146.48kJmol-1
Since the reaction is exothermic, energy is released when this reaction occurs, showing that the reactants energy is lowered when they are converted to products. This shows that the ammonia is lower in energy than the reactants, therefore is more stable than the reactants. However, overall the most stable molecule is the N2 molecule as it has the most negative energy out of N2, H2 and NH3.
S2
Disulfur Molecule |
Optimising S2
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -796.32599779 a.u. |
| RMS Gradient Norm | 0.00000372 a.u. |
| Point Group | D*H |
S-S Bond Length: 1.92943Å
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000011 0.001800 YES RMS Displacement 0.000016 0.001200 YES
The optimisation file is liked to here
Vibrational Analysis

The table to the right shows one mode of vibration. This is consistent with what would be expected as S2 is a diatomic molecule with two atoms so it has (3x2)-5 = 1 vibrational modes. The mode is at 697.03cm-1 which corresponds to a simple stretching mode. This frequency is much lower than that for hydrogen and nitrogen. The reason for this is because the mass of the disulfur molecule is much higher than that of the other two molecules; it is in period 3 of the periodic table rather than period 2.
Charge Analysis
Disulfur is a diatomic consisting of one type of atom. For this reason, neither of the atoms in the molecule have a charge as they both have the same electronegativy.
S2 Molecular Orbitals
MO 7: This molecular orbital is soley the 2pz orbitals on the sulfur atoms. These orbitals are nonbonding as they are too low in energy to be involved in any bonding. It has energy, E = -5.96135 a.u. In the MO diagram there are six MOs of energy around -5.96a.u. to -5.94a.u. These all correspond to0 the in phase and out of phase 2p atomic orbitals - the 2px and 2pz orbitals are degenerate and the 2py orbitals are only 0..1a.u. higher in energy.
MO 14: This molecular orbital is a pi bonding orbital formed from contributions from the 3px orbitals on the sulfur atoms. Is is of energy -0.36284a.u.
MO 16: This molecular orbital is the HOMO. It is a pi antibonding molecular orbital. It is formed from out of phase 3px orbitals on the sulfur atoms. It has an energy of -0.21842 a.u.
MO 17: This molecular orbital is the LUMO. It is a pi antibonding molecular orbital. It is formed by contributions from the out of phase 3py orbitals on the sulfur atoms. It has an energy of -0.18463a.u.
MO 18: This molecular orbital is a σ* orbital (sigma antibonding)molecular orbital. It is formed by the out of phase contributions of the two 3pz orbitals on the sulfur atoms. It has an energy of -0.01305 a.u. The energy of this antibonding orbital is much higher than that of the antibonding orbitals from the other 3p orbitals. This is because it is a σ* rather than a π* molecular orbital. σ* orbitals are higher in energy because the overlap of the atomic orbitals that form the molecular orbitals overlap more for the 3pz orbitals which point directly towards each other than the 3px and 3py orbitals which point parallel to each other. The greater the overlap, the greater the combination of the atomic orbitals, meaning the sigma antibonding orbital is raised to a higher energy than the pi antibonding orbitals due to the contributing orbitals overlapping more effectively.