Rep:Mod:01360107
NH3 molecule
| Molecule | Calculation Method | Basis Set | Final Energy (a.u.) | RMS Gradient (a.u.) | Point Group | Bond Length (Å) | Bond Angle |
|---|---|---|---|---|---|---|---|
| NH3 | RB3LYP | 6-31G(d,p) | -56.55776873 | 0.00000485 | C3V | 1.01798 | 105.741° |
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
NH3 |
According to the 3N-6 rule, NH3 should have 6 modes, as it is a 4 atom molecule.
Modes 2-3 and 5-6 are degenerate.
Modes 1, 2, and 3 are bond bend vibrations, and modes 4, 5, and 6 are bond stretch vibrations.
Modes 1 and 4 are both highly symmetrical.
Mode 1 is called the umbrella mode.
2 bands would be seen in an experimental spectrum of gaseous ammonia.
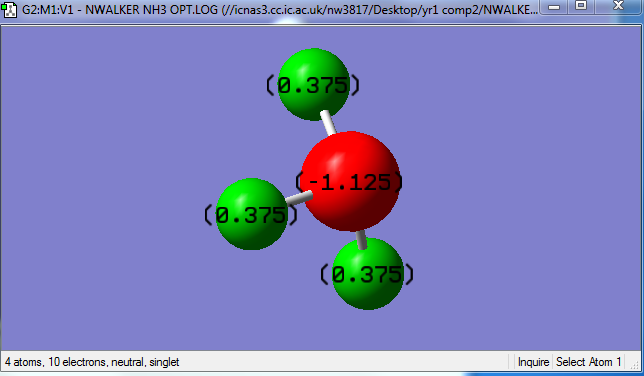
The charge on the Nitrogen was found to be -1.125 and the charges on the Hydrogen were 0.375 for each. This is the kind of result that could be expected, given that Nitrogen is a more electron withdrawing element than Hydrogen, so it would have a higher electron density, it gains a negative charge and the hydrogen become positive.
N2 Molecule
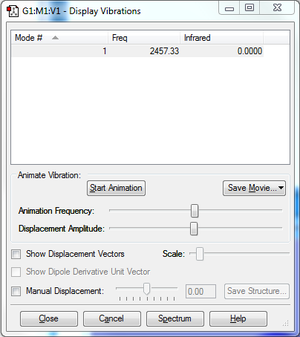
Since there is no net change in dipole moment during the vibration, it will not create a peak in the absorption spectrum, thus the intensity of 0.00 on the table.
| Molecule | Calculation Method | Basis Set | Final Energy (a.u.) | RMS Gradient (a.u.) | Point Group |
|---|---|---|---|---|---|
| N2 | RB3LYP | 6-31G(d,p) | -109.52412868 | 0.00000004 | D*H |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 |
H2 Molecule
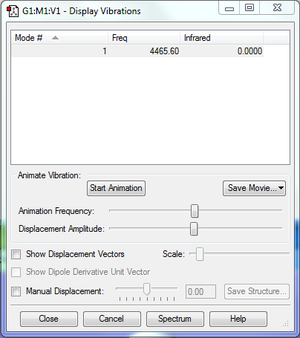
Since there is no net change in dipole moment during the vibration, it will not create a peak in the absorption spectrum, thus the intensity of 0.00 on the table.
| Molecule | Calculation Method | Basis Set | Final Energy (a.u.) | RMS Gradient (a.u.) | Point Group |
|---|---|---|---|---|---|
| H2 | RB3LYP | 6-31G(d,p) | -1.17853936 | 0.00000222 | D*H |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000005 0.001800 YES RMS Displacement 0.000007 0.001200 YES
H2 |
Energies
Energies (atomic units): N2 + 3*H2 -> 2*NH3 E(NH3)= -56.55776873 2*E(NH3)= -113.11553746 E(N2)= -109.52412868 E(H2)= -1.17853936 3*E(H2)= -3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 ΔE= -146.48 kJ/mol
Given the negative ΔE, The reaction is exothermic, so the ammonia product will be more stable than the gaseous reactants.
Experimentally, the enthalpy of formation of 2 moles of ammonia should by -92.38 kJ, however the calculations gave -146.48 kJ, so there is some error in the calculation. [1]
Project Molecule (PH5)
| Molecule | Calculation Method | Basis Set | Final Energy (a.u.) | RMS Gradient (a.u.) | Point Group | Bond Length (Å) | Bond Angle |
|---|---|---|---|---|---|---|---|
| PH5 | RB3LYP | 6-31G(d,p) | -344.25491049 | 0.00000471 | D3H | 1.48687 (axial) or 1.43316 (equatorial) | 90°(axial) or 120° (equatorial) |
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000022 0.001200 YES
PH5 |
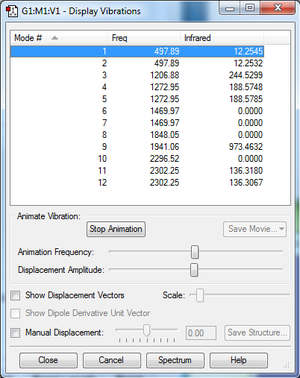
According to the 3N-6 rule, PH5 should have 12 modes, as it is a 6 atom molecule. Modes 1 and 2, 4 and 5, 6 and 7, and 11 and 12 are degenerate. Modes 1-7 are bending vibrations, and modes 8-12 are bond stretch vibrations. Modes 3, 4, 5, 7, 8, 10, 11, and 12 have symmetry. The experimental spectrum of PH5 would give 5 bands.
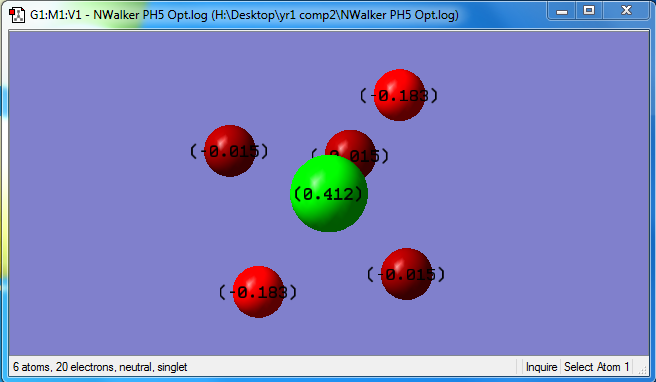
The phosphorus will have a charge of 0.412, the hydrogen in equatorial positions have a charge of -0.015, and hydrogen in axial positions have a charge of -0.183. This is because hydrogen has a slightly greater electronegativity than phosphorous so each hydrogen will slightly withdraw electrons from the central phosphorous, giving it a slightly positive charge and each hydrogen a slightly negative charge.
Molecular Orbitals
MO 6
This is an occupied bonding orbital, comprised of the phosphorus 3s atomic orbital with a contribution from the 2s AO, and the 1s atomic orbitals of each hydrogen. It is a bonding MO, and is low in energy. As a bonding MO, it is stable, and not reactive.
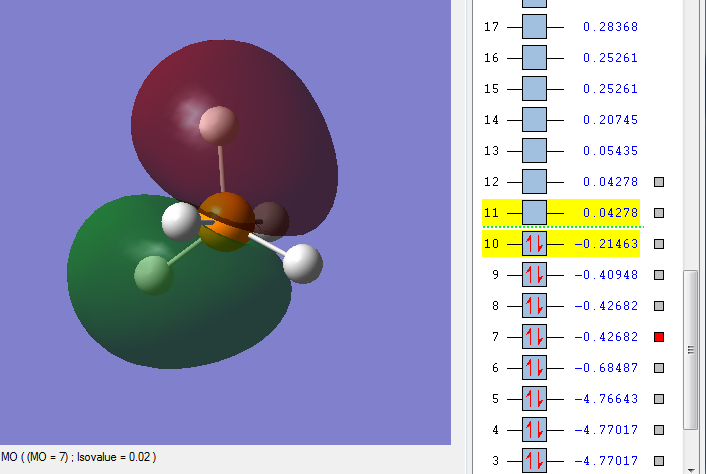
MO 7
This is an occupied bonding orbital, comprised of the phosphorus 3px atomic orbital with a contribution from the 2px AO, and a mix of the 1s and 2s atomic orbitals of 2 equatorial hydrogens. It is a bonding MO, and is relatively low in energy. As a bonding MO, it is stable, and not very reactive.
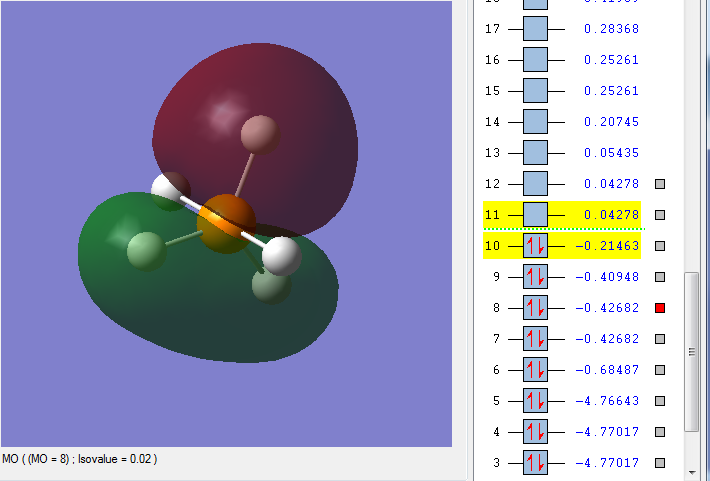
MO 8
This is an occupied bonding orbital, comprised of the phosphorus 3py atomic orbital with a contribution from the 2py AO, and a mix of the 1s and 2s atomic orbitals of the 3 equatorial hydrogens, where the hydrogen directly in line with the phosphorus 3py orbital (in the red area) has a greater contribution than each of the two other equatorial hydrogens. It is a bonding MO, and is relatively low in energy. As a bonding MO, it is stable, and not very reactive.
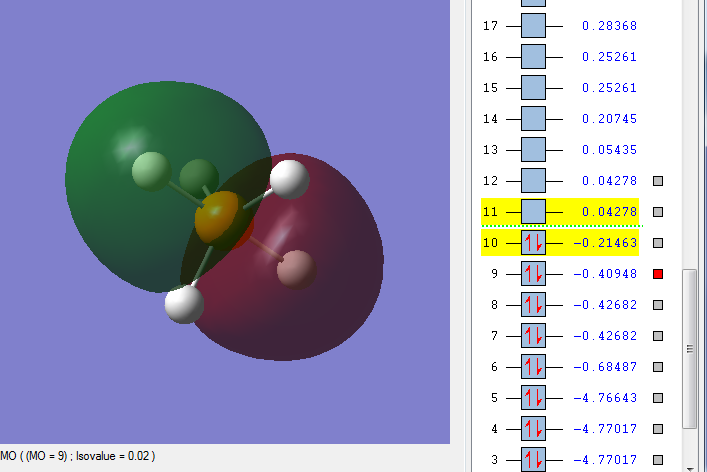
MO 9
This is an occupied bonding orbital, comprised of the phosphorus 3pz atomic orbital with a contribution from the 2pz AO, and a mix of the 1s and 2s atomic orbitals of 2 axial hydrogens. It is a bonding MO, and is relatively low in energy. As a bonding MO, it is stable, and not very reactive.
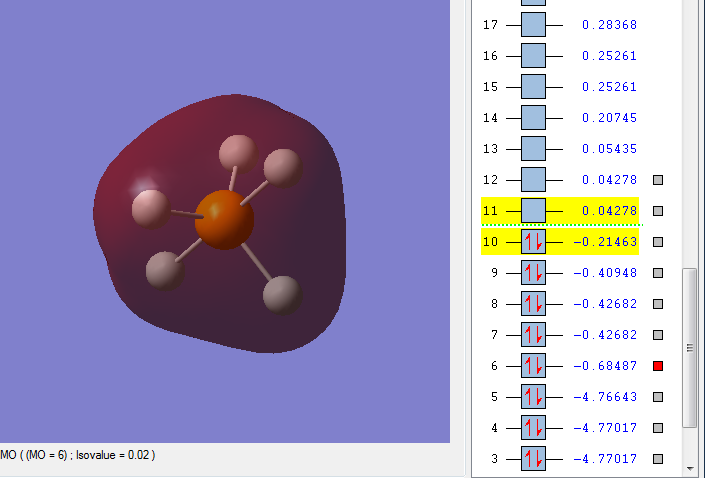
MO10
This is an occupied bonding orbital, comprised of the phosphorus 5dz2 with contribution from the 4s, 5dx2, and 5dy2 atomic orbital and a mix of the 1s and 2s atomic orbitals of each hydrogen, with a greater contribution from the axial hydrogens. It is a bonding MO, and is the highest occupied molecular orbital(HOMO), and therefore in the HOMO energy region. Since the HOMO is a bonding MO, the molecule is very stable, not very reactive.
Comparable Molecule (PF5)
| Molecule | Calculation Method | Basis Set | Final Energy (a.u.) | RMS Gradient (a.u.) | Point Group | Bond Length (Å) | Bond Angle |
|---|---|---|---|---|---|---|---|
| PF5 | RB3LYP | 6-31G(d,p) | -840.67634601 | 0.00010182 | D3H | 1.59656 (axial) or 1.56938 (equatorial) | 90°(axial) or 120° (equatorial) |
Item Value Threshold Converged? Maximum Force 0.000299 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000868 0.001800 YES RMS Displacement 0.000269 0.001200 YES
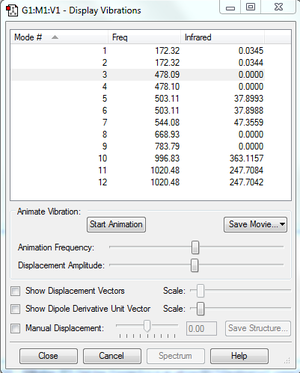
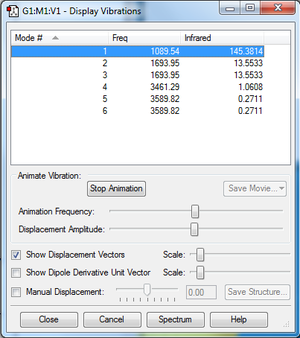
According to the 3N-6 rule, PF5 should have 12 modes, as it is a 6 atom molecule. Modes 1 and 2, 3 and 4, 5 and 6, and 11 and 12 are degenerate. Modes 1-7 are bending vibrations, and modes 8-9 are bond stretch vibrations, and modes 10-12 involve both bond bending and stretching. Modes 3, 4, 5, 7, 8, 9, 10, 11, and 12 have symmetry. The experimental spectrum of PF5 would give 4 bands.
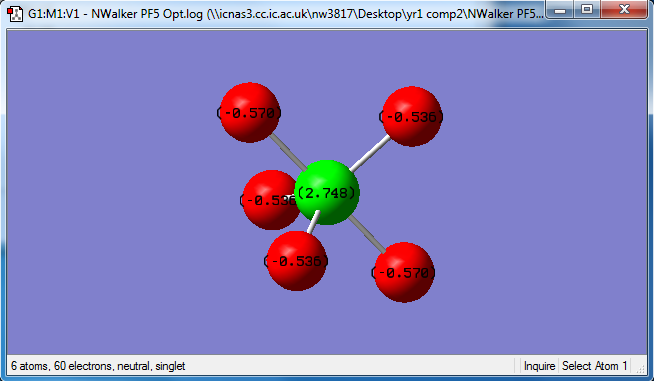
Phosphorous has a charge of 2.748, axial fluorines each have a charge of -0.570 and equatorial fluorines each have a charge of -0.536. This is a similar trend to that in PH5, but the phosphorous has a greater positive charge on it and each fluorine has a greater negative charge than the hydrogens did. This is because fluorine is much more electronegative than hydrogen, so it will withdraw electron density from the central phosphorous much more than the hydrogens did, resulting in greater polarization.
Comparison
As on would expect, PF5 has a similar geometry to PH5, having the same bond angles. It is however, a higher energy molecule, it is polarized to a greater extent due to fluorine's greater electronegativity, and has longer bonds due to Fluorine's greater atomic radius. While it has the same number of vibration frequencies, and many of it's vibration modes are similar to each other, but it's vibrations involve more movement of the central phosphorous, and several of it's modes involve both bond stretching and bond bending which is not the case for PH5.