Rep:Mod:01358709
Molecules
NH3
General information
| Property | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -56.5577683 a.u. |
| RMS gradient | 0.00000485 a.u. |
| Point group | C3v |
| N-H bond length | 1.01798 Å |
| H-N-H bond angle | 105.741˚ |
| Charge(N) | -1.125 |
| Charge(H) | 0.375 |
N has negative charge and H has positive which is to be expected since N is a more electronegative element.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986272D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
File:KERLI TALI NH3 OPTIMIZATION.LOG
Optimized NH3 molecule |
Vibrations
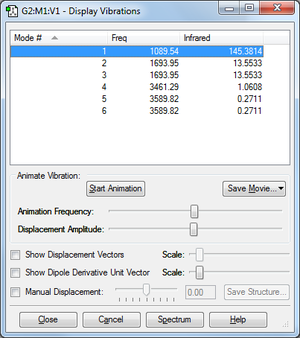
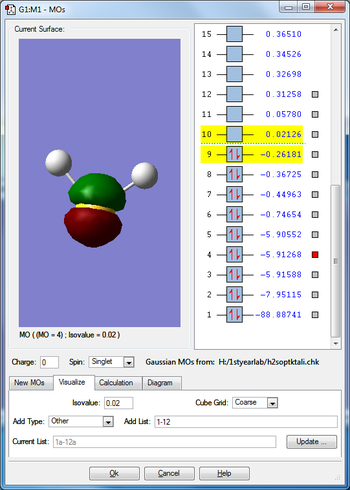
From 3N-6 rule I would expect 3×4-6=6 vibrations, which is how many there are in the table. 1-3 in the table are bending vibrations and 4-6 are stretching vibrations. Modes 2,3 and 5,6 are degenerate. Mode 4 is highly symmetric. Vibration 1 is called the umbrella mode. I would expect to see 3 bands in an experimental spectrum of gaseous ammonia (mode 1, 2&3, 4) because modes 5 and 6 have a very low frequency and these are not likely to be visible due to noise.
N2
| Property | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -109.52412868 a.u. |
| RMS gradient | 0.00000365 a.u. |
| Point group | D*H |
| Bond length | 1.10550 Å |
IR spectra of N2 will not show any bands because N2 is a linear molecule without dipole moment so there is not a vibration that it could undergo that would generate dipole moment.
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Predicted change in Energy=-1.248809D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Optimized N2 molecule |
H2
| Property | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -1.17853864 a.u. |
| RMS gradient | 0.00000204 a.u. |
| Point group | D*H |
| Bond length | 0.74279 Å |
IR spectra of H2 will not show any bands because H2 is a linear molecule without dipole moment so there is not a vibration that it could undergo that would generate dipole moment.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000005 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Predicted change in Energy=-1.634290D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Optimized H2 molecule |
H2S
General information
| Property | Value ! |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -399.39162414 a.u. |
| RMS gradient | 0.00012068 a.u. |
| Point group | C2v |
| Dipole moment | 1.3993 Debye |
| H-S bond length | 1.34737 Å |
| H-S-H bond angle | 92.681˚ |
| Charge(S) | -0.312 |
| Charge(H) | 0.156 |
Charge is positive on H and negative on S which I would expect since S is a more electronegative element.
Item Value Threshold Converged?
Maximum Force 0.000175 0.000450 YES
RMS Force 0.000145 0.000300 YES
Maximum Displacement 0.000472 0.001800 YES
RMS Displacement 0.000386 0.001200 YES
Predicted change in Energy=-1.208488D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.3474 -DE/DX = 0.0002 !
! R2 R(1,3) 1.3474 -DE/DX = 0.0002 !
! A1 A(2,1,3) 92.681 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Optimized H2S molecule |
Vibrations
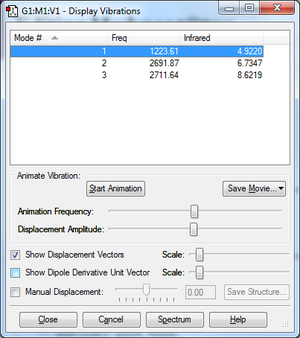
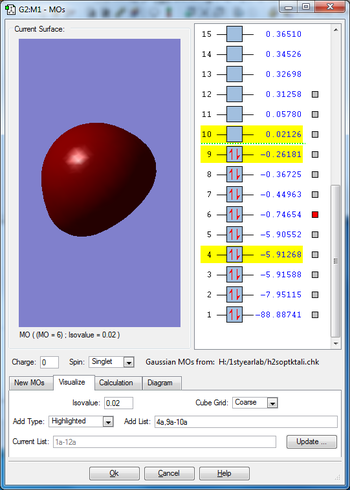
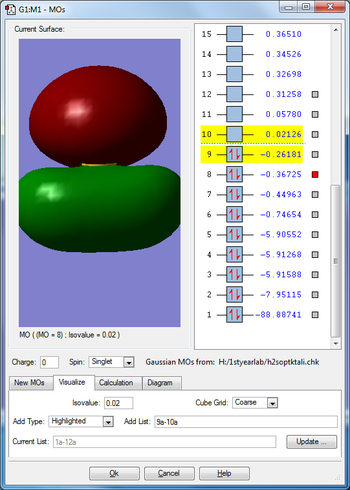
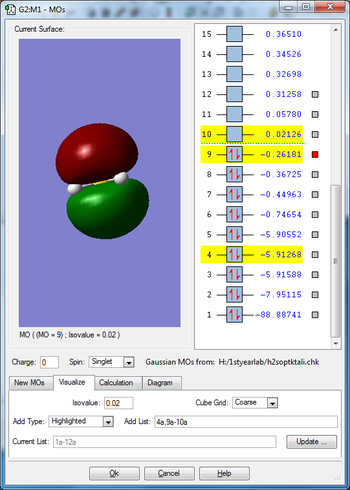
Mode 1 is a bending vibration and 2,3 are bond stretch vibrations. I would expect to see 3 peaks in IR spectra as none of the modes are degenerate.
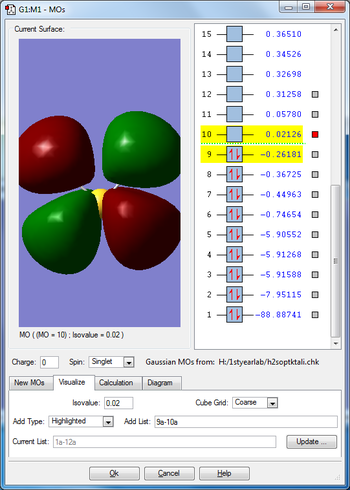
Molecular orbitals
BH3
General information
| Property | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -26.61532362 a.u. |
| RMS gradient | 0.00003234 a.u. |
| Point group | D3H |
| B-H bond length | 1.19219 Å |
| H-B-H bond angle | 120.000˚ |
| Charge(B) | 0.297 |
| Charge(H) | -0.099 |
Item Value Threshold Converged?
Maximum Force 0.000065 0.000450 YES
RMS Force 0.000042 0.000300 YES
Maximum Displacement 0.000254 0.001800 YES
RMS Displacement 0.000166 0.001200 YES
Predicted change in Energy=-2.465372D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1922 -DE/DX = 0.0001 !
! R2 R(1,3) 1.1922 -DE/DX = 0.0001 !
! R3 R(1,4) 1.1922 -DE/DX = 0.0001 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Optimized BH3 molecule |
Vibrations
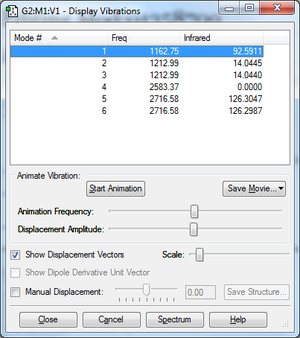
From 3N-6 rule I would expect 6 modes. 1-3 are bending vibrational modes and 4-6 are stretching vibrational modes. 2,3 and 5,6 mode are degenerate. In the experimental spectrum I would expect to see 3 modes (1,2&3,5&6) since mode 4 will not show in IR (no dipole moment change).
Haber-Bosch process
Reaction for Haber-Bosch process is N2 + 3H2 → 2NH3
| Molecule | Energy (a.u.) |
|---|---|
| E(NH3) | -56.5577683 |
| 2*E(NH3) | -113.1155366 |
| E(H2) | -1.17853864 |
| 3*E(H2) | -3.53561592 |
| E(N2) | -109.52412868 |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.0557921 |
ΔE=-146.48 kJ/mol
According to this energy difference, ammonia is more stable than the gaseous reactants.