Rep:Mod:01352190
NH3
| Molecule: | NH3 |
|---|---|
| Calculation method: | RB3LYP |
| Basis Set: | 6-31G (d,p) |
| Final energy (a.u.): | -56.5577687 |
| RMS gradient (a.u.): | 0.00000485 |
| Point group: | C3V |
| Optimised bond length (A): | 1.02 |
| Bond angle (Degrees): | 105.7 |
3-D Structure of Ammonia |
The full log file is available at: File:NH3GAUSSFILE 01352190.LOG
Data generated using GaussView
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986285D-10
Optimization completed.
-- Stationary point found.
NH3 Vibrations
| Mode | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Wavenumber (cm^-1) | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity (arbitrary units) | 145.38 | 13.55 | 13.55 | 1.06 | 0.27 | 0.27 |
Expected number of vibrations (N=6): 3N-6 = 6
modes 2 and 3 are degenerate
modes 5 and 6 are degenerate
1, 2 and 3 are stretching vibrations
4, 5 and 6 are bending vibrations
4 is highly symmetric
2 bands are expected in the spectrum since there are only two energy levels (due to degeneracy)
Charge distribution:
The charge on each hydrogen atom is +0.239 and the charge on the nitrogen atom is -0.717. It is expected that the nitrogen atom has a slight negative charge and each hydrogen atom has a slight positive charge because nitrogen is more electronegative than hydrogen.
N2
N2 Information Summary
| Molecule: | N2 |
|---|---|
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(D,P) |
| Final energy (a.u.): | -109.5241287 |
| RMS gradient (a.u.): | 0.00000060 |
| Point group: | D*H |
| Optimised bond length (A): | 1.11 |
| Bond angle (Degrees): | 180 |
3-D Structure of N2 |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.400972D-13
Optimization completed.
-- Stationary point found.
The full log file is available at: File:N2GAUSSFILE 01352190.LOG
Vibrations
| Mode | 1 |
|---|---|
| Wavenumber (cm^-1) | 2457 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0.00 (not IR active) |
Charge distribution:
There are no partial charges on N2. This is expected since N2 is a homonuclear diatomic molecule.
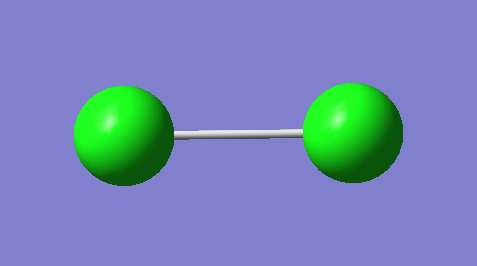
H2
H2 Information Summary
| Molecule: | H2 |
|---|---|
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(D,P) |
| Final energy (a.u.): | -1.1785394 |
| RMS gradient (a.u.): | 0.00000017 |
| Point group: | D*H |
| Optimised bond length (A): | 0.74 |
| Bond angle (Degrees) | 180 |
3-D Structure of H2 |
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
The full log file is available at: File:H2GAUSSFILE 01352190.LOG
Vibrations
| Mode: | 1 |
|---|---|
| Wavenumber (cm-1) | 4466 |
| Symmetry: | SGG |
| Intensity (arbitrary units) | 0.00 (not IR active) |
Charge distribution:
There are no partial charges on H2. This is expected since H2 is a homonuclear diatomic molecule.
The Haber Process Heat Change
N2 + 3H2 -> 2NH3
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= (2*-56.5577687) - (-109.5241287 + (3*-1.1785394)) = -0.0557907 a.u. = -146.5 kJ/mol
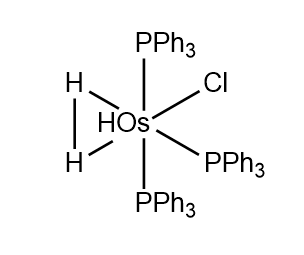
H2 Metal complex
Deposition number: 275803
Database identifier: CEFCAS
H2 bond length = 1.48 A
The H-H bond length is greater in the metal complex than in H-H gas since electron density is donated from the hydrogen atoms to the Osmium atom in the metal complex. This lowers the bond order of the H-H bond, which increases the H-H bond length in the metal complex.
The 3D structure can be found here[1]
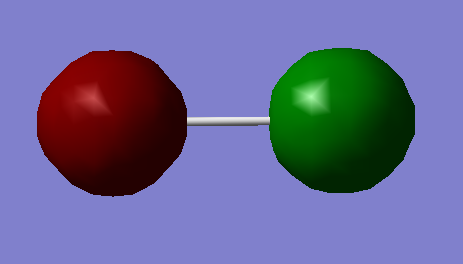
Molecule of choice: Cl2
| Molecule: | Cl2 |
|---|---|
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(D,P) |
| Final energy (a.u.) | -920.3498789 |
| RMS gradient (a.u.) | 0.00002510 |
| Point group: | D*H |
| Optimised bond length (A) | 2.04 |
| Bond angle (Degrees) | 180 |
3-D Structure of Cl2 |
Item Value Threshold Converged?
Maximum Force 0.000043 0.000450 YES
RMS Force 0.000043 0.000300 YES
Maximum Displacement 0.000121 0.001800 YES
RMS Displacement 0.000172 0.001200 YES
Predicted change in Energy=-5.277253D-09
Optimization completed.
-- Stationary point found.
The full log file can be found at: File:CL2GAUSSFILE 01352190.LOG
Vibrations
| Mode: | 1 |
|---|---|
| Wavenumber (cm-1) | 520 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0.00 (not IR active) |
Charge distribution:
There are no partial charges on Cl2. This is expected since Cl2 is a homonuclear diatomic molecule.
Cl2 Orbitals
Atomic orbital contributions: 1s
The molecular orbital is a bonding orbital
The molecular orbital is deep in energy
The molecular orbital is occupied by two electrons
The molecular orbital increases the bond order since it is occupied bonding orbital.
Atomic orbital contributions: 2s
The molecular orbital is an antibonding orbital
The molecular orbital is deep in energy
The molecular orbital is occupied by two electrons
The molecular orbital decreases the bond order since it is an occupied antibonding orbital
Atomic orbital contributions: 2s
The molecular orbital is a bonding orbital
The molecular orbital is deep in energy
The molecular orbital is occupied by two electrons
The molecular orbital increases the bond order since it is an occupied bonding orbital
Atomic orbital contributions: 3p
The molecular orbital is a bonding orbital
The molecular orbital is in the HOMO LUMO region
The molecular orbital is occupied by two electrons
The molecular orbital increases the bond order because it is an occupied bonding orbital
Atomic orbital contributions: 3p
The molecular orbital is an antibonding orbital
The molecular orbital is the LUMO
The molecular orbital does not affect the bond order because it is unoccupied
Extension: O2
| Molecule: | O2 |
|---|---|
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(D,P) |
| Final energy (a.u.) | -150.2574243 |
| RMS gradient (a.u.) | 0.00007502 |
| Point group: | D*H |
| Optimised bond length (A) | 1.22 |
| Bond angle (Degrees) | 180 |
3-D Structure of O2 |
Item Value Threshold Converged?
Maximum Force 0.000130 0.000450 YES
RMS Force 0.000130 0.000300 YES
Maximum Displacement 0.000080 0.001800 YES
RMS Displacement 0.000113 0.001200 YES
Predicted change in Energy=-1.033738D-08
Optimization completed.
-- Stationary point found.
The full log file can be found at File:O2GAUSSFILE 01352190.LOG
Vibrations
| Mode: | 1 |
|---|---|
| Wavenumber (cm-1) | 1643 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0.00 (not IR active) |
Charge distribution:
There are no partial charges on O2. This is expected since O2 is a homonuclear diatomic molecule.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES - easy to follow layout, well done.
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - your answers to the charges question and most of the vibrational questions are good.
However it is due to the low intensity of vibrations 4, 5 and 6 that you only see two peaks in the IR spectrum - there are still 4 separate energy levels!
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
However you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES - good explanation.
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
No you forgot to answer this question.
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - good explanations on these points.
However you have given a bond angle of 180 for Cl2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Independence 0.5/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - you completed a calculation, well done. However you lost 0.5 for the bond angle again.
Do some deeper analysis on your results so far