Rep:Mod:01339518
Ammonia, Nitrogen and Hydrogen
NH3
| Calculation method | B3LYP | |||
| Basis Set | 6-31G (d,p) | |||
| Final Energy | -56.55776873 a.u | |||
| RMS Gradient | 0.00000485 a.u | |||
| Point Group | C3V | |||
| Bond Angle | 105.741o | |||
| Bond length | 1.01798 Å | |||
| Charge on Nitrogen | -1.125 a.u | |||
| Charge on Hydrogens | 0.375 a.u | |||
The charge on nitrogen is expected to be negative. Nitrogen has a higher electronegativity than hydrogen, therefore the electron density is higher around the nitrogen atom.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986284D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad

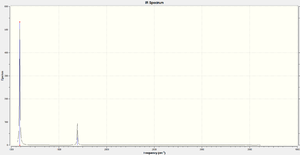
While there are 3 expected bands, 5 and 6 would not be observed in practice, and it is likely 2 and 3 would be at best, difficult to observe. 4 is not observed at all,as there is no change in dipole moment.
| Expected modes of vibration | 6 |
| Degenerate modes | (2,3),(5,6) |
| Bending modes | 1,2,3 |
| Stretching modes | 4,5,6 |
| Most symmetric mode | 4 |
| Umbrella mode | 1 |
| Expected bands | 3 |
H2
| Calculation method | B3LYP | |||
| Basis Set | 6-31G (d,p) | |||
| Final Energy | -1.17853936 a.u | |||
| RMS Gradient | 0.00000017 a.u | |||
| Point Group | D*H | |||
| Bond Angle | 180 o | |||
| Bond length | 0.74279 Å | |||
| Frequency of Vibration | 4465.68 cm-1 | |||
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
File:01339518- H2 OPTIMISATION.LOG
N2
| Calculation method | B3LYP | |||
| Basis Set | 6-31G (d,p) | |||
| Final Energy | -109.52412868 a.u | |||
| RMS Gradient | 0.00000060 a.u | |||
| Point Group | D*H | |||
| Bond Angle | 180 o | |||
| Bond length | 1.10550 | |||
| Frequency of Vibration | 2457.33 cm-1 | |||
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401047D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
File:01339518-N2 OPTIMISATION.LOG
Overall Energy Calculations for the Haber Process
| Energy | Energy / a.u | Energy / kJ mol-1 |
|---|---|---|
| E(NH3) | -56.55776873 | -148492.43 |
| 2.E(NH3) | -113.11553746 | -296984.87 |
| E(N2) | -109.52412868 | -287555.62 |
| E(H2) | -1.17853936 | -3094.26 |
| 3.E(H2) | -3.53561808 | -9282.77 |
| ΔE | -0.05579069 | -146.49 |
The energy change of the reaction is predicted to be -146.49 kJ mol-1, which indicates that it is an exothermic reaction. Therefore the product, NH3, lies lower in energy than that of the reactants H2 and N2
Chosen molecule: CH4
| Calculation method | B3LYP | |||
| Basis Set | 6-31G (d,p) | |||
| Final Energy | -40.52401404 a.u | |||
| RMS Gradient | 0.00003263 a.u | |||
| Point Group | TD | |||
| Bond Angle | 109.471 o | |||
| Bond length | 1.09197 Å | |||
| Charge on Carbon | -0.930 a.u | |||
| Charge on Hydrogens | 0.233 a.u | |||
The bond length of C-H in methane is calculated at 0 Kelvin. Comparisons with experimental literature (1.107 ,1.106 Å)[1][2] and computational literature (1.094 Å)[3] are very similar, considering the effect of temperature causes the bond to extend to a small degree.
| Expected modes of vibration | 9 | ||
| Mode of Vibration | Frequency / cm-1 | Degeneracy | Observed? |
| 1,2,3 | 1356.20 | 3 | Yes |
| 4,5 | 1578.58 | 2 | No |
| 6 | 3046.46 | 1 | No |
| 7,8,9 | 3162.33 | 3 | Yes |
Modes 4, 5 and 6 are not observed as the vibration does not involve a change in dipole moment, therefore it is IR inactive.
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Predicted change in Energy=-2.256035D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.092 -DE/DX = -0.0001 !
! R2 R(1,3) 1.092 -DE/DX = -0.0001 !
! R3 R(1,4) 1.092 -DE/DX = -0.0001 !
! R4 R(1,5) 1.092 -DE/DX = -0.0001 !
! A1 A(2,1,3) 109.4712 -DE/DX = 0.0 !
! A2 A(2,1,4) 109.4712 -DE/DX = 0.0 !
! A3 A(2,1,5) 109.4712 -DE/DX = 0.0 !
! A4 A(3,1,4) 109.4712 -DE/DX = 0.0 !
! A5 A(3,1,5) 109.4712 -DE/DX = 0.0 !
! A6 A(4,1,5) 109.4712 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -120.0 -DE/DX = 0.0 !
! D2 D(2,1,5,3) 120.0 -DE/DX = 0.0 !
! D3 D(2,1,5,4) -120.0 -DE/DX = 0.0 !
! D4 D(3,1,5,4) 120.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Molecular Orbitals of CH4
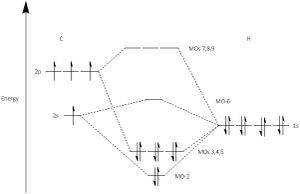
| Molecular Orbital | Orbital Energies / a.u |
| 1 | -10.16707 |
| 2 | -0.69041 |
| 3 | -0.38831 |
| 4 | -0.38831 |
| 5 | -0.38831 |
| 6 | 0.11824 |
| 7 | 0.17677 |
| 8 | 0.17677 |
| 9 | 0.17677 |
| 10 | 0.52915 |
| 11 | 0.52915 |
| 12 | 0.52915 |
The orbital occupations can be seen on figure 2. Note that MO-1 is not on figure 2, as the orbital is so deep in energy that it does not interact with any significance with the H 1s orbitals. It would be parallel to
Bonding and Non Bonding Orbitals
MO-1 is the lowest energy MO of methane, where it is composed of only the 1s orbital of carbon. As the 1s orbital is so deep in energy,it is a core orbital, therefore the interaction of 1s with the H orbitals is minimal. The coefficients of C 1s was 0.99284. It is non bonding. The shape is almost identical of the 1s sphere shape.
MO-2 is formed by the contribution of 2s on carbon and the 1s orbitals of hydrogen. The s character leads to a lower energy than that of the 2p-1s MOs. The orbital coefficients reflect on this, where C 2s and H 1s had values of 0.38672 and 0.13785 respectively.
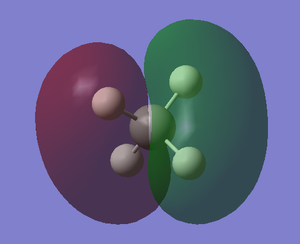
MO's 3, 4 and 5 are degenerate, they are the HOMOs. They are composed of the 3 2p orbitals from carbon and the three 1s orbitals of the hydrogen atoms. The orbital coefficients of C 2p and H 1s were 0.44276 and 0.17016. They are orientated along the axis of the contributing 2p orbital.
MO's 2,3,4 and 5 are bonding orbitals as they are lower in energy than the orbitals that formed them. (H 1s, C 2s and 2p)
| MO-1 | MO-2 (sigma) | MO-4 (sigma) |
 |
 |
|
Anti Bonding Orbitals
MO-7 is a combination of C 2p and 3p with H 2s. The coefficients are 0.38122,1.34476 and ±1.07455.
MO-10 is a combination of C 3p and H 1s and 2s to a smaller but not insignificant degree. The coefficients are 1.45073, ±0.28550 and ±0.14317.
Note that the calculations made by Gaussian for unoccupied orbitals are unreliable, and they are approximations made by placing electrons into the higher orbitals.
| MO-7 (sigma) | MO-10 (sigma) |
 |

|
References
- ↑ The Journal of Chemical Physics33, 1254 (1960); doi: 10.1063/1.1731367
- ↑ The Journal of Chemical Physics35, 1211 (1961); doi: 10.1063/1.1732025
- ↑ 1. Handy, N. C., Murray, C. W. & Amos, R. D. Study of methane, acetylene, ethene, and benzene using Kohn-Sham theory. J. Phys. Chem. 97, 4392–4396 (1993)
