Rep:Mod:01338303
NH3 Molecule
General Information
N-H bond distance = 1.01798 Å
H-N-H bond angle = 105.741°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -56.55776870 a.u.
RMS Gradient Norm: 0.00000485 a.u.
Point Group: C03V
Item Table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Jmol 3D Model
NH3 Molecule |
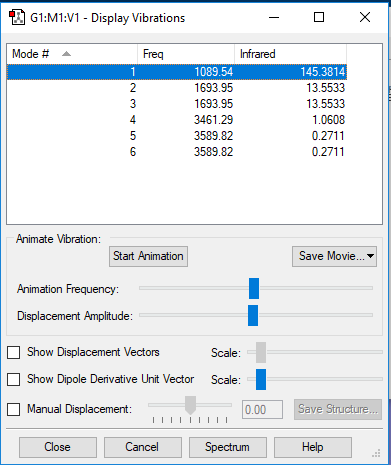
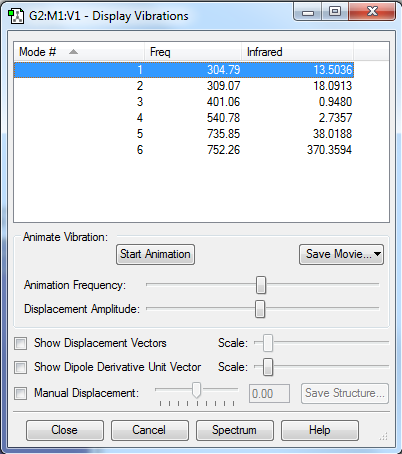
Display Vibrations
Questions about Display Vibrations
1.How many modes do you expect from the 3N-6 rule?
Modes = 3*4-6 = 6
2.Which modes are degenerated (i.e. have the same energy?)
Modes 2&3 and modes 5&6 are degenerated.
3.Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Modes 1&2&3 are bending and modes 4&5&6 are stretching.
4.Which mode is highly symmetric?
Mode 4 is highly symmetric.
5.One mode is known as the "umbrella" mode, which one is this?
Mode 1 is the "umbrella" mode.
6.How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
There are 4 bands.
Charge
The charge on N atom is -1.125.
The charge on H atom is +0.375.
Since N atom is more electronegative than H atom, the charge on N atom is negative while that on H is positive.
H2 Molecule
General Information
H-H bond distance = 0.74279 Å
H-H bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -1.17853936 a.u.
RMS Gradient Norm: 0.09719500
Point Group: D*H
Item Table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Jmol 3D Model
H2 Molecule |
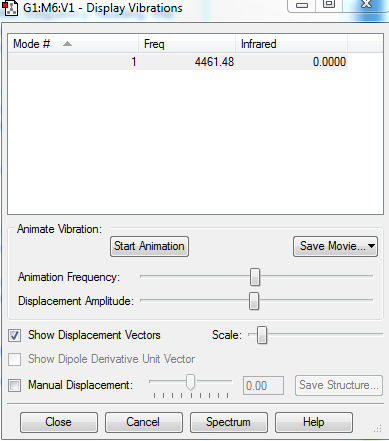
Display Vibrations
N2 Molecule
General Information
N-N bond distance = 1.09200 Å
N-N bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -109.52359111 a.u.
RMS Gradient Norm: 0.02473091 a.u.
Point Group: D*H
Item Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol 3D Model
N2 Molecule |
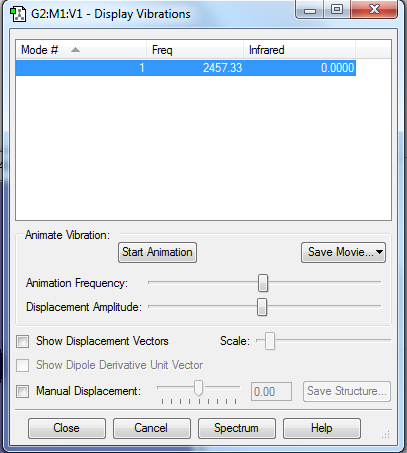
Display Vibrations
Reaction Energies
E(NH3) = -56.55776870 a.u.
2*E(NH3) = -113.1155370 a.u.
E(N2) = -109.52359111 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = -113.1155370+113.05920919 = -0.05632781 a.u. = -147.89 kJ/mol Since the energy difference is negative, the ammonia product is more stable.
Project molecule--F2 Molecule
General Information
F-F bond distance = 1.16000 Å
F-F bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -199.42620785 a.u.
RMS Gradient Norm: 0.23253407 a.u.
Point Group: D*H
Item Table
Item Value Threshold Converged?
Maximum Force 0.000128 0.000450 YES
RMS Force 0.000128 0.000300 YES
Maximum Displacement 0.000156 0.001800 YES
RMS Displacement 0.000221 0.001200 YES
Jmol 3D Model
F2 Molecule |
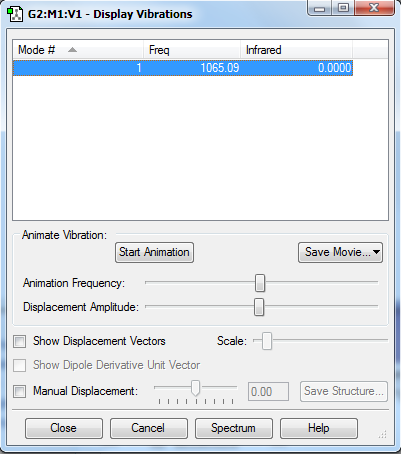
Display Vibrations
Charge
There is the same charge on 2 F atoms and F2 molecule does not carry on a overall charge.
Molecular Orbital
MO1
MO2
MO3
MO4
MO5
Summary
. MO1 and MO2 are bonding and anti-bonding orbitals respectively formed by 2s AOs of F atom. Both of them are occupied and they are sigma bonds.
. 2p AOs of F atom contribute to the formation of MO3, MO4 and MO5. MO3 and MO4 are occupied and bonding while MO5 is unoccupied and anti-bonding. To be specific, MO3 and MO5 are sigma MOs formed by 2 Py AOs and however, MO4 is pi MOs which is formed by 2 Px AOs.
.Since MO3 and MO4 are bonding, they have relatively low energy in HOMO region, at -0.58753 a.u. and -0.52332 a.u.
. Meanwhile, MO5 is the LUMO (Lowest Unoccupied Molecular Orbital) with a relatively low energy (-0.12679 a.u.)
. Bond order of F2 Molecule is 1 so it is less stable than N2 molecule.
Independence--ClF3 Molecule
General Information
Cl-F bond distance = 1.57000 Å
F-Cl-F bond angle = 90° (axial) & 180° (equatorial)
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -759.43206418 a.u.
RMS Gradient Norm: 0.05217279 a.u.
Point Group: C2V
Item Table
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000204 0.001800 YES
RMS Displacement 0.000134 0.001200 YES
Jmol 3D Model
ClF3 Molecule |
Display Vibrations
Charge
Charge on Cl atom: +1.225
Charge on equatorial F atom: -0.316
Charge on axial F atom: -0.454