Rep:Mod:01334475
Haber Process Molecules
NH3
| Molecule | NH3 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -56.55776873 au |
| RMS Gradient | 0.00000485 au |
| Point Group | C3V |
| N-H Bond Length | 1.018 Å |
| H-N-H Bond Angle | 105.7° |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS Force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000072 | 0.001800 | YES |
| RMS Displacement | 0.000035 | 0.001200 | YES |
| Predicted Change in Energy | -5.986267D-10 |
Optimised NH3 |
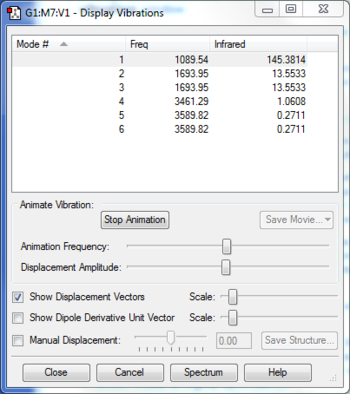
• Using the 3N-6 rule we expect to find 6 modes of vibrations
• Modes 2 and 3 are degenerate, as are modes 5 and 6
• Modes 1, 2 and 3 are all bending vibrations whereas modes 4, 5 and 6 are stretches
• Mode 4 is highly symmetric as all three bonds are stretched simultaneously
• Mode 1 is known as the umbrella mode as all three bonds bend in the same direction simultaneously, resembling an umbrella opening.
• You would expect to see 3 bands in an experimental spectrum as this is number of asymmetrical, non-degenerate modes of vibration
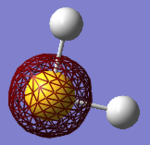
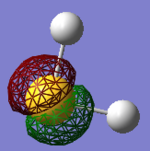
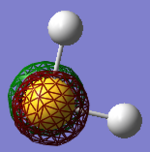
• You would expect the nitrogen atom to be negatively charged and the hydrogen atoms to be positively charged due to the large difference in electronegativity. The relative charge distributions are as expected, the charge on N is -1.125 and whereas the H atoms have charges of +0.375
H2
Optimised H2 |
| Molecule | H2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -1.17853936 au |
| RMS Gradient | 0.00000017 au |
| Point Group | D*H |
| H-H Bond Length | 0.7428 Å |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000000 | 0.000450 | YES |
| RMS Force | 0.000000 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000001 | 0.001200 | YES |
| Predicted Change in Energy | -1.164080D-13 |
| Mode | Frequency | Infrared |
|---|---|---|
| 1 | 4465.68 | 0.0000 |
N2
Optimised NH3 |
| Molecule | N2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -109.52412868 au |
| RMS Gradient | 0.00000060 au |
| Point Group | D*H |
| N-N Bond Length | 1.106 Å |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000001 | 0.000450 | YES |
| RMS Force | 0.000001 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000000 | 0.001200 | YES |
| Predicted Change in Energy | -3.400919D-13 |
| Mode | Frequency | Infrared |
|---|---|---|
| 1 | 2457.33 | 0.0000 |
Energy Calculations
| E(NH3) | -56.55776873 au |
| 2*E(NH3) | -113.11553746 au |
| E(N2) | -109.52412868 au |
| E(H2) | -1.17853936 au |
| 3*E(H2) | -3.53561808 au |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.0557907 au |
• ΔE = -0.0557907 au = -146.478 kJmol-1
• The haber process reaction is exothermic which suggests that the products (NH3) are more stable than the reactants (H2 and N2). From the calculated energy difference we see that this is true since we have a negative value.
Project Molecules
H2S
| Molecule | H2S |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -399.39162414 au |
| RMS Gradient | 0.00012068 au |
| Point Group | C2V |
| H-S Bond Length | 1.347 Å |
| H-S-H Bond Angle | 92.68° |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000175 | 0.000450 | YES |
| RMS Force | 0.000145 | 0.000300 | YES |
| Maximum Displacement | 0.000472 | 0.001800 | YES |
| RMS Displacement | 0.000386 | 0.001200 | YES |
| Predicted Change in Energy | -3.400919D-13 |
Optimised NH3 |
| Mode | Frequency | Infrared |
|---|---|---|
| 1 | 1223.61 | 4.9220 |
| 2 | 2691.87 | 6.7347 |
| 3 | 2711.64 | 8.6219 |

• There are 3 expected vibrational modes by using the 3N-6 rule calculation
• Mode 1 is a bend whereas the other two modes are stretches
• There are no degenerate modes, therefore there are three expected peaks on the IR spectrum
• Mode 2 is a highly symmetric stretch
• The sulfur has a relative charge of -0.312 and the hydrogens each have a relative charge of +0.156. This is due to sulfur having greater electronegativity which means that there is more electron density concentrated around it.
• MOs 1-5 are not involved in bonding since they are too deep in energy to be accessed. They consist of Sulfur's atomic orbitals from 1s to 2p
• MO 6 is a bonding orbital consisting of Sulfur's 3s orbital and the σ MO from H2. MOs 7 and 8 are similar in that they form due to mixing of Sulfur's AOs and the σ MO from H2. However instead of a 3s, MOs 7 and 8 arise from 3p AOs.
• MO 9 is not involved in bonding and is a single 3p orbital from Sulfur.
HCl
Optimised NH3 |
| Molecule | HCl |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -460.80077875 au |
| RMS Gradient | 0.00005211 au |
| Point Group | C*V |
| H-Cl Bond Length | 1.286 Å |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000090 | 0.000450 | YES |
| RMS Force | 0.000090 | 0.000300 | YES |
| Maximum Displacement | 0.000139 | 0.001800 | YES |
| RMS Displacement | 0.000197 | 0.001200 | YES |
| Predicted Change in Energy | -1.256951D-08 |
| Mode | Frequency | Infrared |
|---|---|---|
| 1 | 2956.80 | 18.0303 |