Rep:Mod:01196775
NH3 molecule
nh3 optimisation File Name ZoharMS_nh3_optf_pop File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -56.55776873 a.u. RMS Gradient Norm 0.00000485 a.u. Imaginary Freq 0 Dipole Moment 1.8466 Debye Point Group C3V Job cpu time: 0 days 0 hours 0 minutes 9.0 seconds.
The optimised N-H bond length was 1.01798 Å, in contrast with a literature value of 1.008Å[1], a difference likely due to the fact that electronegativity differences between nitrogen and hydrogen mean that the bond has some ionic character and is therefore slightly shorter and stronger than can be predicted by an optimisation based on a completely covalent structure.
The optimised H-N-H bond angle was 105.741°, compared with a literature value of 107.298°[1]. This could be due to a small discrepancy in modelled electron density versus in real conditions.
Despite small differences, the calculated values for bond length and angle are a close fit to the literature values, implying that Gaussview is able to very accurately model these molecules. Therefore, energy calculations based on this are likely to be accurate for the conditions under which the molecule is modelled.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986298D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
optimised NH3 |
The optimisation file is linked to here
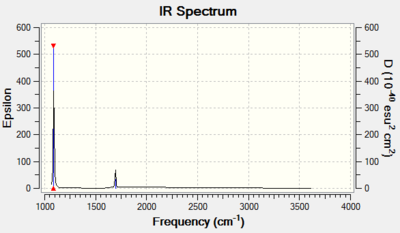
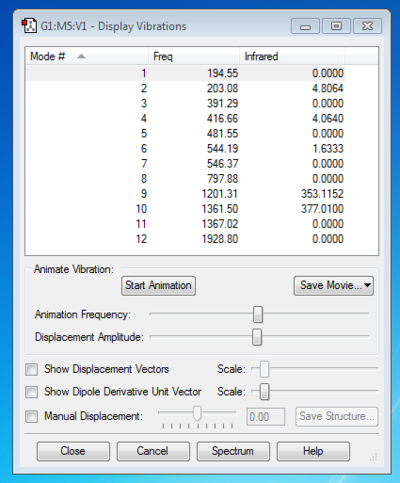
From the 3N-6 rule, 6 vibrations were expected and subsequently calculated.
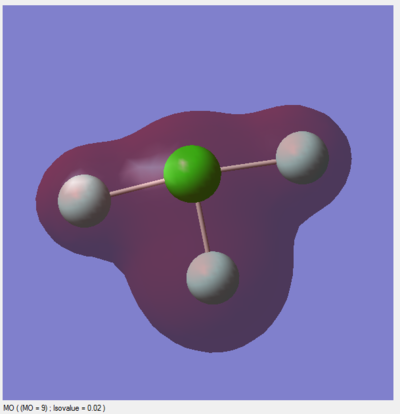
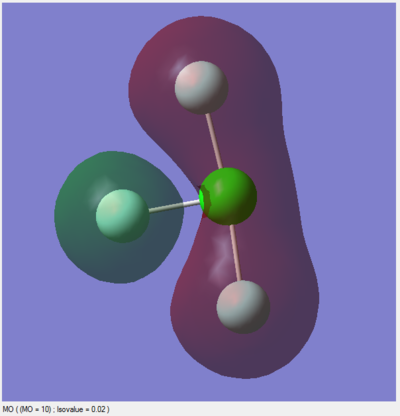
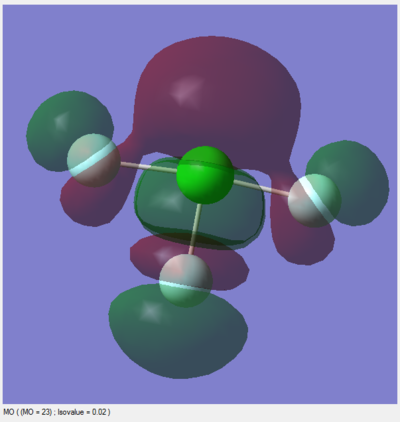
Modes 2 & 3 as well as 5 & 6 are degenerate, due to their having the same IR frequency, and therefore the same energy as frequency is proportional to energy by the de Broglie relationship. Modes 1, 2, 3 are bending vibrations while 4, 5, 6 are bond stretching vibrations. Mode 4 is highly symmetric as all the N-H bonds contract and extend in unison. Mode 1 is an umbrella mode since the N-H bonds move upwards around the nitrogen atom like an umbrella opening.
In an experimental IR spectrum of gaseous ammonia, one could expect to see 2 bands as only three of the vibrations (1,2,3) have a change in dipole moment and therefore a high enough intensity on IR to be visible, and two of these (2,3) are degenerate and thus will only produce a single band.
There is a charge of -1.125 on nitrogen and +0.375 on each of the hydrogens. This is as expected as nitrogen is more electronegative than hydrogen, so it will usually hold bonding electrons more tightly than hydrogen in an N-H bond. Additionally, there is a lone pair of electrons on nitrogen with a significant negative charge.
N2 molecule
n2 optimisation File Name ZoharMS_n2_optf_pop File Type .chk Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(D,P) Charge 0 Spin Singlet Total Energy -109.52412868 a.u. RMS Gradient Norm 0.00000365 a.u. Imaginary Freq Dipole Moment 0.0000 Debye Point Group D∞h
Bond length: 1.10550 Å
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Predicted change in Energy=-1.248809D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
optimised N2 |
The optimisation file is linked to here
As can be seen below, N2 has only one mode with an IR spectrum of intensity 0. This is due to the fact that charge is equally distributed between both nitrogens; therefore no dipole is present and no change in dipole moment is possible.
H2 molecule
h2 optimisation File Name ZoharMS_h2_optf_pop File Type .chk Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(D,P) Charge 0 Spin Singlet Total Energy -1.17853936 a.u. RMS Gradient Norm 0.00000017 a.u. Imaginary Freq Dipole Moment 0.0000 Debye Point Group D∞h
Bond length: 0.74279 Å
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
optimised H2 |
The optimisation file is linked to here
H2 produces a blank IR spectrum for the same reasons as N2 does: it is homonuclear and so no change in dipole moment is possible.
Energy changes associated with the Haber-Bosch process
The Haber-Bosch process is an important chemical process by which ammonia is formed artificially from nitrogen and hydrogen gases. It is carried out on a massive scale globally as it provides a significant proportion of the ammonia used in fertilisers, and has therefore enabled the production of sufficient food for the growing global population. The energy associated with this process is important as it is quite energy intensive and understanding the energy associated with this exothermic reaction can aid in the design of reactors in which to carry out the process, and in the design of industrial reaction conditions.
Energies of each of these molecules were obtained by optimisation in Gaussview 5.0, and analysed in the following manner in hartree units:
E(NH3)= -56.55776873
2*E(NH3)= -113.11553746
E(N2)= -109.52412868
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907
This is equivalent to a ΔE of -146.47849401 kJ/mol calculated for the formation of 2NH3, so the calculated enthalpy of formation of ammonia is -73.239247005kJ/mol. The literature value for the enthalpy of formation of ammonia is -45.910 kJ/mol[2], showing a significant difference between the calculated value and the actual value. This difference can be attributed to a difference in the conditions at which the experiment was conducted, such as the medium and temperature, as well as the compounding of small discrepancies in real and calculated bond lengths and angles.
ClF3 molecule
ClF3 T shape optimisation File Name ZoharMs_ClF3_Tshape_optf_pop File Type .chk Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(D,P) Charge 0 Spin Singlet Total Energy -759.46531688 a.u. RMS Gradient Norm 0.00002465 a.u. Imaginary Freq Dipole Moment 0.8386 Debye Point Group
Optimised Cl-F bond lengths were 1.72863 Å with the outer fluorines and 1.65143 Å with the middle fluorine, compared with literature values of 1.698 Å and 1.598 Å[3], which is a fairly good agreement; again, electronegativity making the bonding have a small degree of ionic character is likely the cause.
The optimised F-Cl-F bond angle was 87.140°, compared with a literature value of 87.29°[3], a very close agreement. This angle is not 90° as in a typical trigonal bipyramidal structure as the two lone pairs repel the bonding pairs by a greater degree than a pair of bonding electrons would have.
Originally these values were optimised for a ClF3 molecule in a trigonal planar structure, but the results were significantly different to the literature values (all three Cl-F bonds were of length 1.75684 Å) and so an alternative T-shape structure for three bonding pairs and two lone pairs was investigated, which gave better agreement with the literature values.
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000204 0.001800 YES
RMS Displacement 0.000134 0.001200 YES
Predicted change in Energy=-1.250234D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.6514 -DE/DX = 0.0 !
! R2 R(1,3) 1.7286 -DE/DX = 0.0 !
! R3 R(1,4) 1.7286 -DE/DX = 0.0 !
! A1 A(2,1,3) 87.1404 -DE/DX = 0.0 !
! A2 A(2,1,4) 87.1404 -DE/DX = 0.0 !
! A3 L(3,1,4,2,-1) 174.2807 -DE/DX = 0.0 !
! A4 L(3,1,4,2,-2) 180.0 -DE/DX = 0.0 !
! D1 D(1,2,4,3) 0.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
optimised ClF3 |
The optimisation file is linked to here
The charge on chlorine is +1.225 and that on the two outermost fluorine atoms is -0.454, while the charge on the fluorine in the middle of the T shape is -0.316. Fluorine carries a negative charge and chlorine a positive charge as fluorine is more electronegative and polarizes electron density towards itself. The middle fluorine is not as negatively charged as the other two as the pseudostructure is trigonal bipyramidal and the middle Cl-F bonding pair experiences less electronic repulsion, as it has a greater angle between it and the lone pairs than the outer fluorines do.
This is the lowest energy bonding MO, and its contributing AOs are 2s on chlorine and 2s on all three fluorine atoms. It is relatively deep in energy (-1.28161), it is occupied and it contributes roughly homogenous electron density across all four atoms. This is the lowest energy MO despite being made up of 2s orbitals as the 1s orbitals are too low in energy and are therefore non-bonding.
This is a part-bonding, part-antibonding occupied orbital deep in energy (-1.17368) with all contributions coming from 2s AOs. Its effect is to increase electron density between chlorine and the two peripheral fluorines as it will undergo positive interference with the first MO shown here. It also decreases electron density between chlorine and the middle fluorine due to the node present between them.
This is a bonding, occupied MO below the HOMO/LUMO region in energy (-0.53818), with electron density contributed by 2p AOs on all atoms. It has electron density above and below the plane of the molecule but not within it, implying some π character (though a later π* orbital must cancel this out as the overall bonding in the molecule is primarily σ).
This is the HOMO and therefore its energy is in the HOMO/LUMO region (-0.33458). It is occupied but antibonding as it can be seen that it consists of 2p orbitals on each of the atoms which are perpendicular to the plane of the molecule, which are all out of phase with each other. It is interesting to note the smaller 2p orbital size on the middle fluorine, which earlier was shown to have a less negative charge than the other two fluorines. This MO is the reason why π bonding is not observed in this molecule, as it consists of multiple π* orbitals.
This is an antibonding LUMO made up of 2p orbitals on chlorine and all three fluorines, therefore it is unoccupied. These 2p orbitals are involved in creating the σ* orbitals, and therefore lie in the plane of the molecule. It can be seen that it is completely antibonding as there is a node across each bond in the molecule. Its energy is in the HOMO/LUMO region (-0.15086) and if it is occupied, this will cause all Cl-F bonds to break.
Tetrafluoroethene: the monomer of PTFE
Polytetrafluoroethene (PTFE) is a material with very low friction and is therefore useful for coating pans to make them non-stick. It is a polymer of tetrafluoroethene (TFE) monomers and so understanding the energy requirements for synthesising TFE is useful for industry. The following is an investigation of the structure of TFE.
TFE optimisation File Name ZOHARMS_TFE_OPTF_POP File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -475.49961623 a.u. RMS Gradient Norm 0.00006120 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group C2H Job cpu time: 0 days 0 hours 0 minutes 22.0 seconds.
There was an optimised C-F bond length of 1.32423 Å[4] compared with a literature range of 1.311–1.321 Å, which the optimised value is just above. The optimised C=C bond length of 1.32540 Å was compared with the literature value of 1.337 Å[4], a close agreement.
The optimised F-C-F bond angle was 113.716°, compared with the literature value of 104.35°[4]. This agreement was less close and could have been more strongly influenced by differences in assumed conditions when modelling versus in the real experiment, including medium and temperature.
The charge on both the carbon atoms was 0.631 and on all the fluorines was -0.315 as the molecule is symmetrical and fluorine is more electronegative than carbon, therefore drawing electron density towards itself in each C-F bond.
Item Value Threshold Converged?
Maximum Force 0.000206 0.000450 YES
RMS Force 0.000067 0.000300 YES
Maximum Displacement 0.000222 0.001800 YES
RMS Displacement 0.000146 0.001200 YES
Predicted change in Energy=-5.785176D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.3254 -DE/DX = 0.0002 !
! R2 R(1,3) 1.3242 -DE/DX = 0.0 !
! R3 R(1,4) 1.3241 -DE/DX = 0.0001 !
! R4 R(2,5) 1.3242 -DE/DX = 0.0 !
! R5 R(2,6) 1.3241 -DE/DX = 0.0001 !
! A1 A(2,1,3) 123.1336 -DE/DX = 0.0 !
! A2 A(2,1,4) 123.1507 -DE/DX = 0.0 !
! A3 A(3,1,4) 113.7158 -DE/DX = 0.0 !
! A4 A(1,2,5) 123.1336 -DE/DX = 0.0 !
! A5 A(1,2,6) 123.1507 -DE/DX = 0.0 !
! A6 A(5,2,6) 113.7158 -DE/DX = 0.0 !
! D1 D(3,1,2,5) 180.0 -DE/DX = 0.0 !
! D2 D(3,1,2,6) 0.0 -DE/DX = 0.0 !
! D3 D(4,1,2,5) 0.0 -DE/DX = 0.0 !
! D4 D(4,1,2,6) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
optimised TFE |
The optimisation file is linked to here
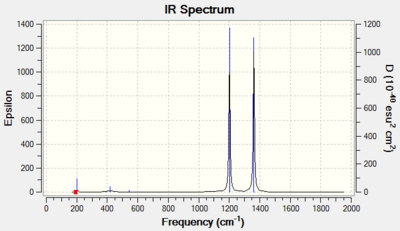
The IR spectrum frequencies are shown below. As can be seen from the intensities, one could expect two peaks in the diagnostic region as the rest of the vibrations either have no change in dipole or only a small change in dipole which would produce extremely small peaks.
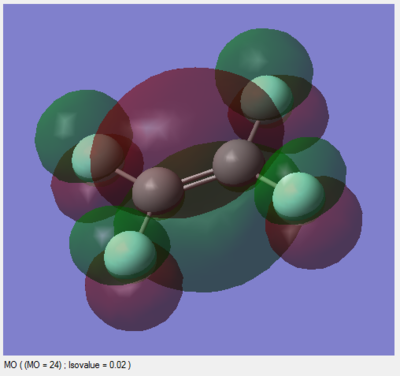
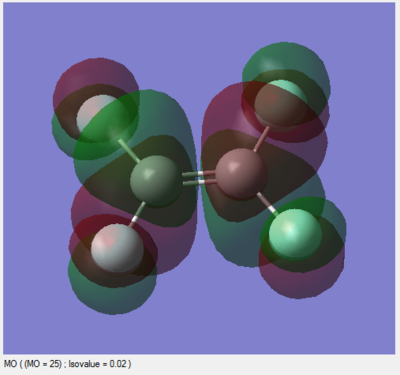
Below, two molecular orbitals are investigated: the HOMO and LUMO of TFE. The LUMO is of particular interest as it is the interactions between the LUMOs of multiple TFE monomers which cause them to react to form PTFE.
This is the HOMO with an energy of -0.25422. It has both bonding and antibonding character and is the final filled orbital, which serves to increase electron density in the C=C π bond above and below the plane of the molecule by positive interference with lower energy orbitals which also have bonding character between the carbon atoms. It also reduces electron density between each carbon and fluorine due to the presence of a node in the C-F bonds. It is made up of the 2p orbital perpendicular to the plane of the molecule on each atom, and is therefore unaffected by the sp2 hybridisation of carbon (the hybrid orbitals lie in the plane of the molecule).
This is the LUMO. It is similar to the HOMO in terms of which AOs are involved, but its energy is positive (0.03000) and it is completely antibonding. The electron density on each fluorine is smaller than that in the HOMO implying slightly weaker antibonding character in the C-F bond, but importantly there is a node between the two carbon atoms representing the antibonding C=C π*. If electron density is donated into this LUMO by another TFE molecule, the negative interference between the HOMO (C=C π) and LUMO (C=C π*) causes the C=C π bond to break and only the C-C σ bond remains. The electrons now enter two new C-C σ bonds with neighbouring monomers to form the polymer.
References
- ↑ 1.0 1.1 Table of Interatomic Distances and Configuration of Molecules and Ions, Special Publication No 11; Supplement 1956–1959, Special Publication No 18, Chemical Society, London, 1958, 1965.
- ↑ M. Sana, G. Leroy, D. Peeters and C. Wilante, J. Mol. Struct., 1988, 164, 249-274.
- ↑ 3.0 3.1 D.F. Smith, J. Chem. Phys., 1953, 21(4), 609–614.
- ↑ 4.0 4.1 4.2 D. Lentz, A. Bach, J. Buschmann, P. Luger and M. Messerschmidt, Chem. Eur. J., 2004, 10, 5059 - 5066.