Rep:Mod:01196400
NH3 Molecule
Ammonia (NH3) Properties:
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final Energy = -56.55776873 a.u.
RMS gradient = 0.00000485 a.u.
Point group = C3V
N-H Bond length = 1.01798 Angstrom
H-N-H Bond Angle = 105.741°
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 Jmol
Ammonia Molecule |
Vibrational Modes
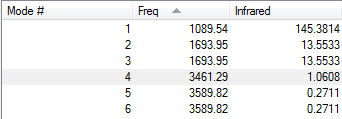
The 3N-6 rule would suggest 6 vibrational mode.
Modes 2 & 3 and 5 & 6 are degenerate.
Modes 1,2 and 3 are bending modes. 4, 5 and 6 are stretching modes.
Mode 4 is highly symmetric. Mode 1 is the "umbrella" mode.
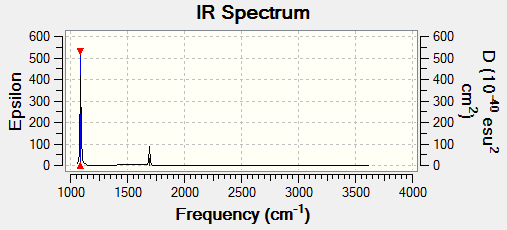
You would expect to see 4 bands in an experimental spectrum of gaseous ammonia.
The peaks at 3461.26cm-1 and 3589.82cm-1 have negligible intensities and are therefore not seen on the spectrum.
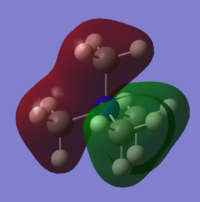
Charge Distribution
The charge on the hydrogen is expected to be positive and the charge on the nitrogen negative.
The calculated values are:
• -1.125 on N
• 0.375 on H
Haber-Bosch Process
H2 Molecule
Hydrogen (H2) Properties:
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final Energy = -1.17853936 a.u.
RMS gradient = 0.00000017 a.u.
Point group = DinfH
H-H Bond length = 0.74279 Angstrom
H-H Bond Angle = 180°
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 Jmol
Hydrogen Molecule |
Vibrational Modes
None negative.
Charge Distribution
Purely covalent bond therefore no charge on the hydrogen atoms.
N2 Molecule
Nitrogen N2 Properties
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final Energy = -109.52412868 a.u.
RMS gradient = 0.00000060 a.u.
Point group = DinfH
N-N Bond length = 1.10550 Angstrom
N-N Bond Angle = 180°
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 Jmol
Nitrogen Molecule |
Vibrational Modes
None negative.
Charge Distribution
Purely covalent bond therefore no charge on the nitrogen atoms.
Reaction Energies
N2 + 3H2 -> 2NH3
•E(NH3)= -56.55776873 a.u.
•2*E(NH3)= -113.11553746 a.u.
•E(N2)= -109.52412868 a.u.
•E(H2)= -1.17853936 a.u.
•3*E(H2)= -3.53561808 a.u.
•ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -113.11553746 - (-109.52412868 - 3.53561808) = -0.05579069 a.u.
-0.05579069 Hartrees = -146.48 kJmol-1
The ammonia product is therefore lower in energy than the gaseous reactants. The product is more stable than the reactants.
CH4 Molecule
Structure
Methane (CH4) Properties:
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final Energy = -40.52401404 a.u.
RMS gradient = 0.00003263 a.u.
Point group = Td
C-H Bond length = 1.09197 Angstrom
H-C-H Bond Angle = 109.471°
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES
CH4 Jmol
Methane molecule |
Vibrational Modes
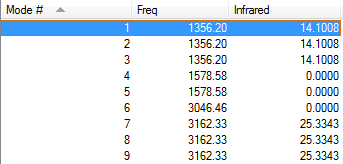
The 3N-6 rule would suggest 9 vibrational modes.
Modes 1, 2 & 3 are degenerate. As are 4 & 5 and 7, 8 & 9.
1, 2, 3, 4, 5 & 6 are bending modes. 6, 7, 8 & 9 are stretching modes.
Mode 6 is highly symmetric.
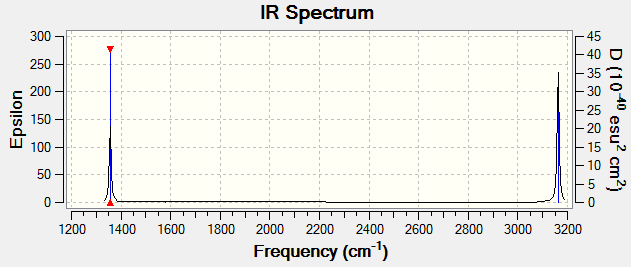
You would expect to see 2 peaks in an experimental spectrum of gaseous methane.
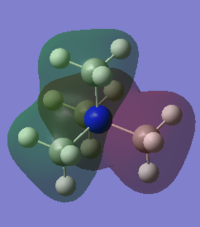
Charge Distribution
You would expect the carbon to have a negative charge and the hydrogen a positive charge.
Calculated values:
•Carbon = -0.930
•Hydrogen = 0.233
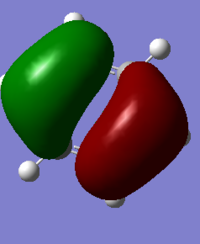
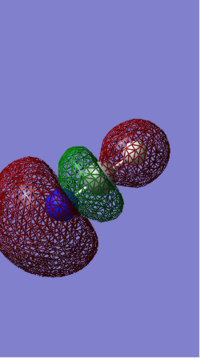
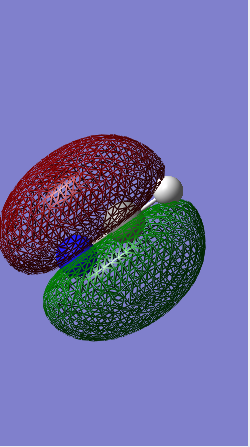
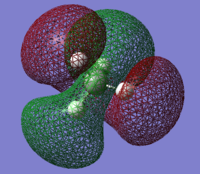
CH4 Molecular Orbitals
MO 1
This MO is very deep in energy (-10.16707 Hartrees).
This is a non-bonding orbital and therefore has no contribution to the bonding of the structure. It is from the 1s orbital of the carbon.
MO 2
This MO is deep in energy (-0.69041 Hartrees).
This is a bonding orbital formed from the hybridization of the hydrogen's 1s orbitals and the carbon's 2s.
Since this MO is both bonding and occupied it'll have a large effect on the bonding in methane.
MOs 3, 4 & 5
These MOs are all degenerate with an energy of -0.38831 Hartrees.
They are bonding orbitals from the hybridization of the 2p orbitals in the carbon and the 1s of the hydrogens.
Since these MOs are both bonding and occupied they'll have a large effect on the bonding in methane.
This is methanes HOMO.
MO 6
This has a high energy of 0.11824 Hartrees.
It is the anti bonding orbital formed from the hybridization of the 2s on carbon and 1s of the hydrogens.
This orbital is unoccupied and so it doesn't have an effect on the bonding in methane.
It is the LUMO.
MOs 7, 8 & 9
These MOs are degenerate and high in energy, 0.17677 Hartrees.
They are anti bonding orbitals formed from the hybridization of the 2p orbitals on the carbon and the 1s of the hydrogens.
These orbitals are unocuppied and so they don't have an effect on the bonding in methane.