Rep:Mod:01190874
Annie Rouse, CID=01190874
NH3
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
The energy was calculated to be: -56.55776873 (au)
RMS Gradient: 0.00000485
Point Group: C3V
Bond distance: 1.01798 angstrom
Bond angle: 105.741 degrees
The table showing that the calculations converged is below:
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Ammonia |
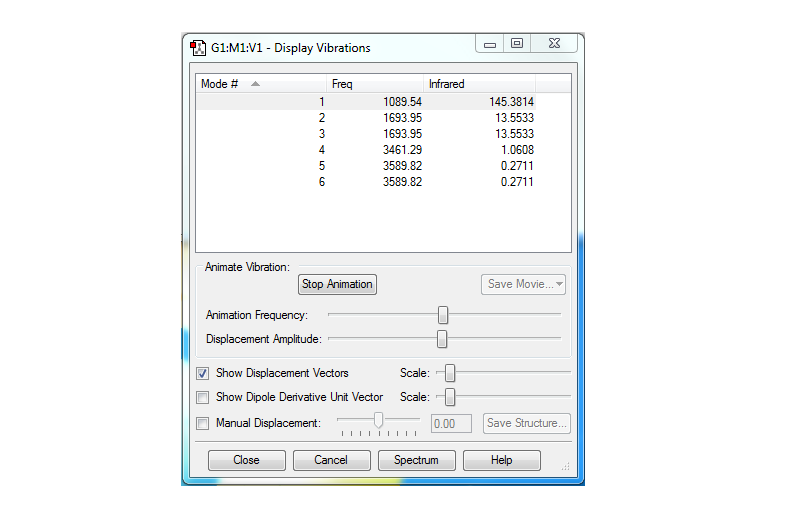
Below is a screenshot showing the vibrational modes calculated for the molecule.
From the 3N-6 rule, 6 modes are expected and 6 are observed, since N is 3.
There are 2 pairs of degenerate modes (2 and 3, 5 and 6).
1,2 and 3 are bending and 4, 5 and 6 are stretching.
The 4th mode is highly symmetric (ie. the symmetric stretch mode) whereas modes 5 and 6 are asymmetric stretches.
Umbrella mode is the 1st mode (when the molecule opens and closes like an umbrella - this is a bending mode)
2 bands expected in experimental IR, since there are 2 distinct energies at which a change in dipole moment would be induced - one of the pairs of degenerate modes is not IR active as it does not induce a change in dipole moment (modes 5 and 6), the first pair of degenerate modes only produces one band since, and the 4th mode (highly symmetric) does not induce a change in dipole moment and thus would not produce a band in the IR spectrum.
The charges on the atom were calculated as follows: H has charge 0.375; N has charge -1.125. This is in accordance with expected results since N is more electronegative than H and thus would be expected to bear more negative charge in a compound with only H.
N2
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
The final energy was calculated to be: -109.52412868 (au)
RMS Gradient Norm: 0.00000060
Point group: Dinfh
Bond length: 1.10550 angstrom Bond angle: 180 degrees
The table showing the calculations converged is below:
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
There is only one vibrational mode, a stretching mode at 2457.33 cm-1.
This is in accordance with the 3N -5 rule (since N is 2). The 3N -5 rule is used since this molecule is linear.
The charges on each atom are 0 since this is a homonuclear diatomic and there is no difference in electronegativity.
H2
Calculation Method: RB3LYP
Basis Set: 6-31G(D,P)
E(RB3LYP): -1.17853936 (au)
RMS Gradient Norm: 0.00000017
Point group: Dinfh
Bond length: 0.74279 angstrom
Bond angle: 180 degrees
The table showing that the calculations converged is below:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
There is only one vibrational mode, a stretching mode at 4465.68 cm-1
This is in accordance with the 3N -5 rule (this rule is used since the molecule is linear), and since N is 2.
The charges on the atoms are 0 since this is a homonuclear diatomic and there is no difference in electronegativity.
Reaction Energy Calculations
In order to find the reaction enthalpy for the Haber-Bosch process: N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873
2*E(NH3)= -113.115537
E(N2)= -109.52412868
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579024 (au)
Thus the reaction enthalpy of the Haber-Bosch process is -146.48 kJmol-1 (the result in au above was multiplied by 2625.5 to convert it into kJmol-1).
Thus the product (N3) is more stable than the reactants (N2 and H2), as the energy change is negative and the product is lower in energy than the reactants.
A literature value for the enthalpy of the formation of ammonia (essentially what has been calculated above) is −46.22 kJ/mol[1] The difference between the enthalpy calculated here and the literature value could be attributed to several different factors, particularly that the optimised energies for the molecule are theoretical only and depend on the calculation method use, and therefore may not represent accurately what is physically observed in reality.
H2O
H2O
Point group: C2V
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
The final energy was calculated to be: -76.41973740 (au)
RMS Gradient Norm: 0.00006276
Bond length: 0.96522 angstrom
Bond angle: 103.745 degrees
The table showing the calculations converged is below:
Item Value Threshold Converged? Maximum Force 0.000099 0.000450 YES RMS Force 0.000081 0.000300 YES Maximum Displacement 0.000128 0.001800 YES RMS Displacement 0.000120 0.001200 YES
Water |
The charge on the H atoms is: 0.472
Charge on the O atom: -0.944
The charges make sense, since O is more electronegative than H.
There are 3 vibrational modes, which is in accordance with the 3N-6 rule (used since this molecule is non linear, and N is 3).
The first, at 1665.00 cm-1 is a symmetrical bending mode.
The second, at 3801.05 cm-1 is a symmetrical stretch.
The third, at 3914.23 cm-1 is an asymmetric stretch.
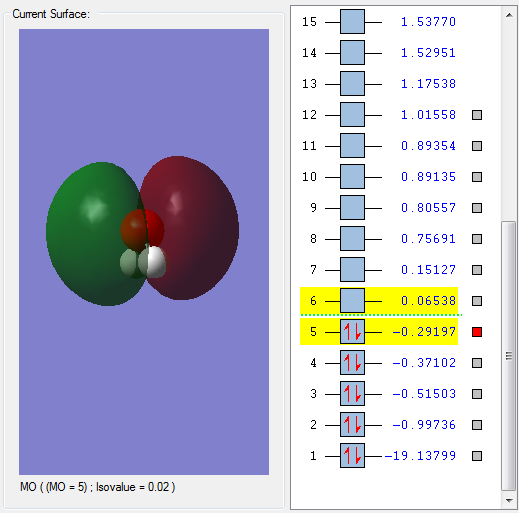
This is a sigma orbital formed by overlap of the 1s orbitals on the H atoms, and the 2s orbital on an O atom. It is deep in energy (the deepest energy bonding orbital) and is fully occupied. There are no nodes across bonds and thus this is a bonding orbital.
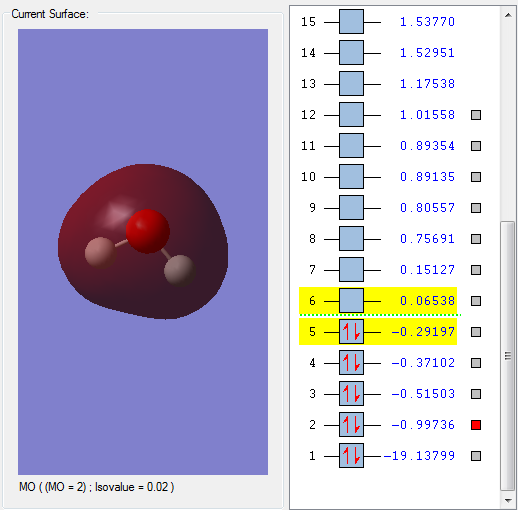
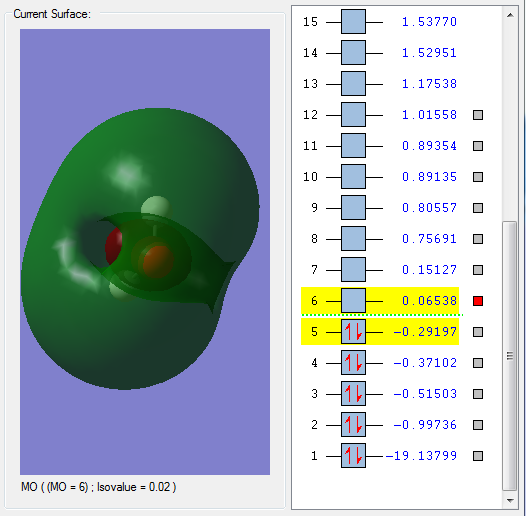
This is a sigma orbital formed by overlap of a 2p orbital on the O atom and the 1s orbitals on the H atoms. It is fully occupied, and is an bonding orbital, as the node is across the O atom not the O-H bonds. It is evident that this MO is formed by overlap of a 2p orbital and a 1s orbital because the node across the O atom indicates a p orbital is involved. It is quite deep in energy.
This orbital is a non-bonding orbital accounting for the lone pair characteristics of the O on water. It is fully occupied, and is the last MO to be fully occupied - this is the HOMO. There is negligible contribution from the 1s orbitals on the H atoms, so this orbital has mostly p character.
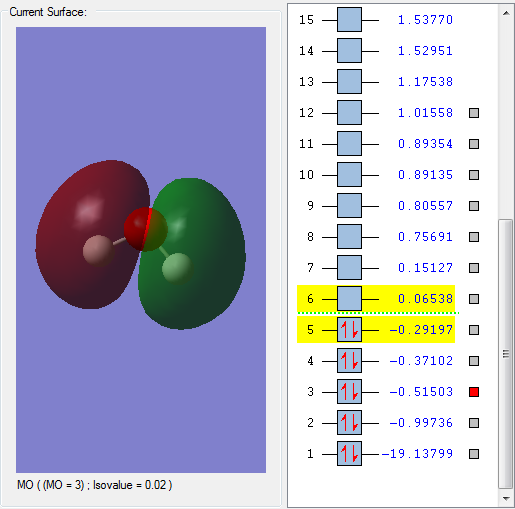
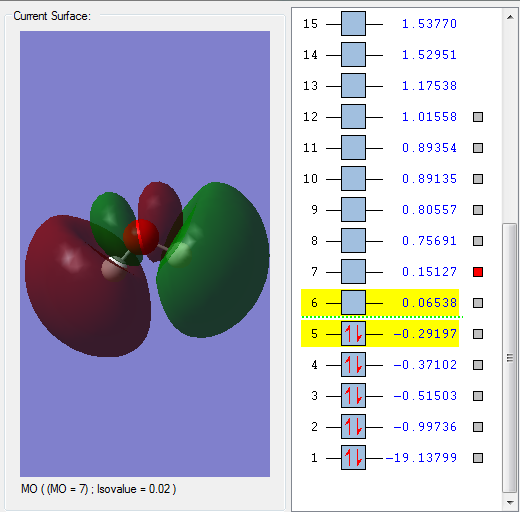
This orbital is formed by overlap of a 2p orbital on the O atom and the 1s orbitals on the H atoms. It is the first unoccupied MO and thus this is the LUMO, and is an antibonding orbital as there are nodes across the O-H bonds. It contributes to the reactivity of water, as if another species attacks a water molecule, it interacts with this MO.
This orbital is formed by overlap of a 2p orbital on the O atom and the 1s orbitals on the H atoms. It is an antibonding orbital, as there are nodes across the O-H bonds, and is relatively high in energy (positive) and thus doesn't contribute a great deal to the bonding in H2O.
- ↑ Appl, M. 2006. Ammonia. Ullmann's Encyclopedia of Industrial Chemistry.