Rep:Mod:01188090
BH3
Item Value Threshold Converged? Maximum Force 0.000047 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.000183 0.001800 YES RMS Displacement 0.000092 0.001200 YES
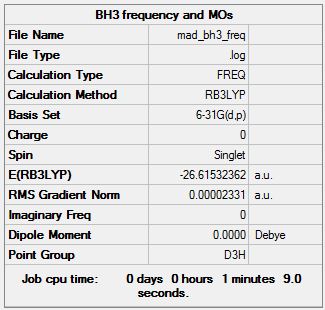
Frequency analysis log file MAD_BH3_FREQ.LOG
Smf115 (talk) 08:21, 17 May 2018 (BST)Links to the required log files are broken throughout for each structure.
Low frequencies --- -0.4059 -0.1955 -0.0054 25.3481 27.3325 27.3356 Low frequencies --- 1163.1913 1213.3139 1213.3166
BH3 |
| wavenumber (cm-1) | intensity (arbitrary units) | symmetry | IR active? | type |
|---|---|---|---|---|
| 1163 | 93 | A1 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |

There are less than 6 peaks in the IR spectrum, despite the presence of 6 vibrational modes in the table as not all the modes lead to an overall change in dipole moment. For a mode to be IR active, there needs to be a change in dipole moment across the molecule, and when a molecule of BH3 symmetrically stretches, all three bonds stretch at the same time, thus the dipole moment of the molecule doesn't change.
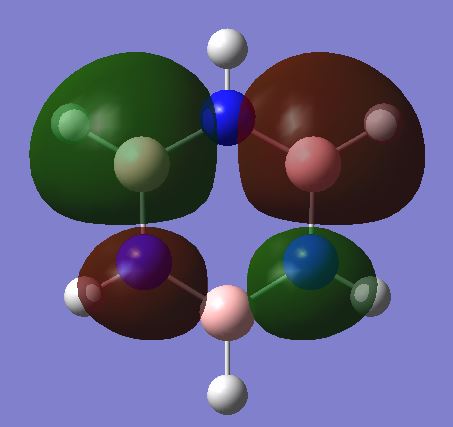
Molecular orbital diagram for BH3

The MOs generated computationally do not differ significantly from the LCAO MOs, showing that qualitative MO theory can be used as a reasonably accurate way of predicting the shapes of MOs.
NH3
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
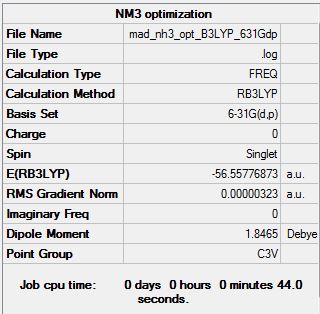
Frequency analysis log file MAD_NH3_FREQ.LOG
Low frequencies --- -11.6527 -11.6490 -0.0045 0.0333 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
NH3 |
NH3BH3
Item Value Threshold Converged? Maximum Force 0.000153 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000465 0.001800 YES RMS Displacement 0.000241 0.001200 YES
Frequency analysis log file MAD_NH3BH3_FREQ.LOG
Low frequencies --- -0.1516 -0.0504 -0.0205 11.4593 17.9090 18.0876 Low frequencies --- 263.1437 631.4267 638.8923
NH3BH3 |
E(NH3) = -56.55776873 au E(BH3) = -26.61532362 au E(NH3BH3) = -83.22469115 au
ΔE = (E(NH3)+E(BH3)) - E(NH3BH3) =(-56.558 + -26.615) - (-83.224) = 0.051 au =130 ± 10 kJ mol-1 (lit. 130.1 ± 4.2 kJ mol-1)1
Upon comparison with a literature value for the dissociation energy of a normal B-H bond (377.9 ± 8.7 kJ mol-1)1, it is shown that the bond energy calculated for a dative B-N bond is relatively weak in comparison.
BBr3
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000056 0.001800 YES RMS Displacement 0.000028 0.001200 YES
Low frequencies --- -0.0132 -0.0064 -0.0046 2.5123 2.5124 4.8831 Low frequencies --- 155.9619 155.9639 267.6983
BBr3 |
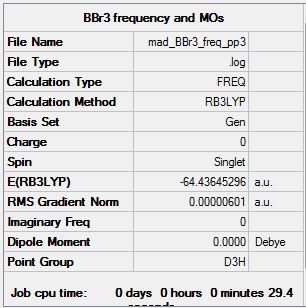
Frequency analysis log file Mad_BBr3_freq_opt_pp5.log
Benzene C6H6
Item Value Threshold Converged? Maximum Force 0.000202 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000769 0.001800 YES RMS Displacement 0.000326 0.001200 YES
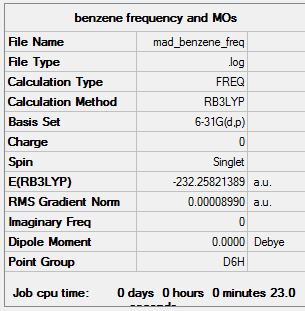
Frequency analysis log data MAD_BENZENE_FREQ.LOG
Low frequencies --- -2.5530 -2.5530 -0.0088 -0.0041 -0.0039 10.3930 Low frequencies --- 413.9723 413.9723 621.1358
Benzene |
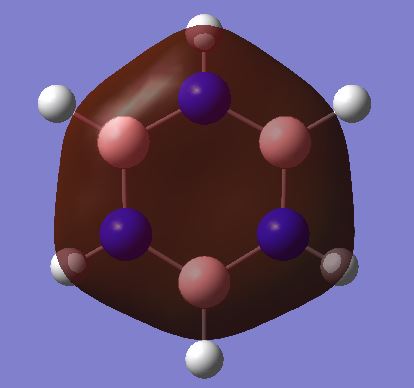
Borazine B3H6N3
Item Value Threshold Converged? Maximum Force 0.000093 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.000241 0.001800 YES RMS Displacement 0.000099 0.001200 YES
Frequency analysis data MAD_BORAZINE_FREQ.LOG
Low frequencies --- -10.7286 -10.4463 -10.2311 -0.0104 -0.0093 0.0981 Low frequencies --- 289.0927 289.1014 403.7381
Borazine |
Benzene vs. Borazine
Charge distribution comparison
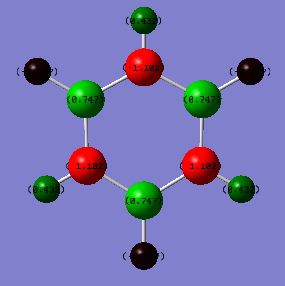
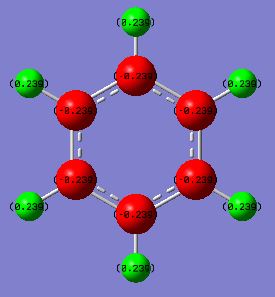
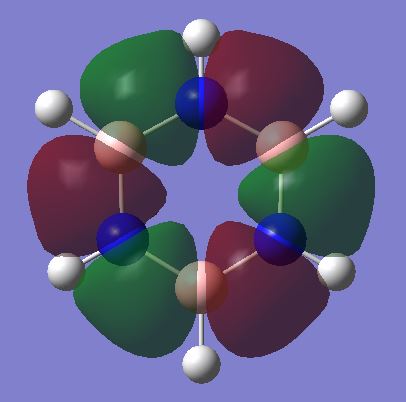
Benzene shows a uniform charge density across the six carbon atoms due to the high symmetry that benzene possesses. The six carbon atoms in benzene have a charge of -0.239 and the six hydrogen atoms have a charge of 0.239. As the hydrogen atoms are attached to more electronegative carbon atoms, the carbon atoms will have a higher electron density as the electrons are drawn more towards the carbon atoms. In borazine, the six-membered ring is comprised of alternating boron and nitrogen atoms, and has less symmetry in comparison to benzene. Nitrogen has a higher electronegativity than boron, therefore there is higher electron density on the nitrogen atoms in comparison with the boron atoms. This is shown through the respective charges calculated, 0.747 for boron and -1.103 for nitrogen. The hydrogen atoms bonded to boron have a higher electron density than those attached to nitrogen atoms, as those attached to nitrogen are attached to the more electronegative atom, and as a result the electron density will be pulled away from hydrogen and onto nitrogen. In comparison, the boron atoms are not as electron withdrawing, thus the hydrogen atoms attached to boron will have a higher electron density in comparison with those attached to nitrogen.
Smf115 (talk) 08:21, 17 May 2018 (BST)Good charge anaysis with considertion to both symmetry and electronegativities of the atoms.
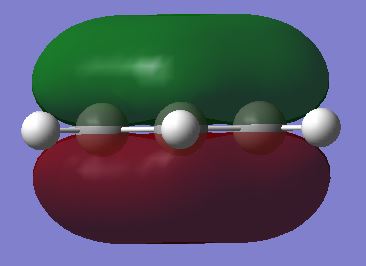
Molecular orbital comparison
Aromaticity
It is generally accepted that there are 4 criteria that need to be fulfilled in order to describe a molecule as aromatic: the molecule must be cyclic, the molecule must be planar, the molecule must have fully conjugated p orbitals on every atom in the system and the molecule must have 4n+2 pi electrons.3
However these rules can be called into contention with a few examples. Firstly the simple model commonly used to portray aromatic compounds of overlapping pz atomic orbitals is not an accurate description of aromaticity in aromatic compounds. Although these overlapping orbitals form a molecular orbital in benzene, this is only one of many molecular orbitals that arise. Other molecular orbitals involve combinations of p and s atomic orbitals combining to give other molecular orbitals. Furthermore, in cases such as borazine where the cyclic system is comprised of heteroatoms, the polarity differences between the heteroatoms is not taken account in the given rules and as a result, this leads to molecular orbitals with less symmetry, than for those in benzene.
A further observation made that can discredit the above set of rules for aromaticity is that not all aromatic molecules are in fact planar. Good examples of systems that show aromatic characteristics but which are not planar are para- and meta- cyclophanes.4 Even at very low temperatures, benzene is observed to adopt a chair conformation instead of a planar conformation due to strong intermolecular forces present when crystal lattices form.4
Smf115 (talk) 08:16, 17 May 2018 (BST)Good references to examples which contradict the planar concept for aromaticity. Further discussion and consideration towards MOs and how the concept of overlapping pZ AOs is a bad descriptor of aromaticity would have improved the answer.
Smf115 (talk) 08:20, 17 May 2018 (BST)Overall a decent and well presented wiki report.
References
[1] - https://notendur.hi.is/agust/rannsoknir/papers/2010-91-CRC-BDEs-Tables.pdf
[2] - http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
[3] - Clayden Organic chemistry
[4] - https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.200700250