Rep:Mod:01181216201805
Inorganic Computational Lab
EX3 Section
BH3
1. B3LYP/3-21G Calculation Method
Method and Basis Set: B3LYP/3-21G
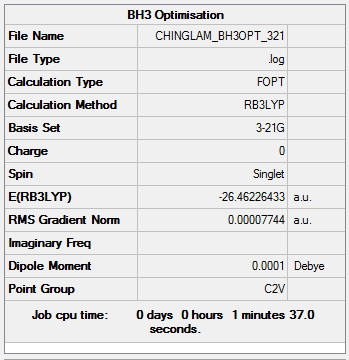
Optimization log file: CHINGLAM BH3OPT 321.LOG
Item Table
Item Value Threshold Converged? Maximum Force 0.000174 0.000450 YES RMS Force 0.000100 0.000300 YES Maximum Displacement 0.000674 0.001800 YES RMS Displacement 0.000397 0.001200 YES
Borane |
Smf115 (talk) 00:02, 26 May 2018 (BST)Nice to see the 3-21G calculation shown, it would be good to mention between this and the 6-31G(d,p) calculation about why the basis set was improved (this is only additional information, but worth considering).
2. B3LYP/6-31G(d,p)Calculation Method
Method and Basis Set: B3LYP/6-31G(d,p)
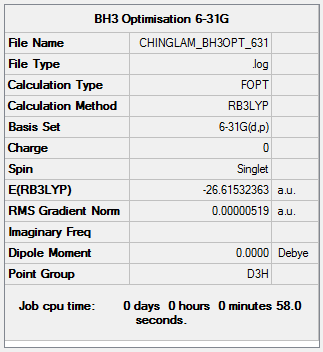
Optimization log file: CHINGLAM BH3OPT 631.LOG
Frequency analysis log file: CHINGLAM BH3OPT 631 FREQ.LOG
Item Table
Item Value Threshold Converged? Maximum Force 0.000010 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000041 0.001800 YES RMS Displacement 0.000027 0.001200 YES
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- -4.8294 -1.2074 -0.0054 1.0243 9.1094 9.1890 Low frequencies --- 1162.9789 1213.1709 1213.1736
Jmol Image
Borane |
IR Vibrations
| Mode | wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
|---|---|---|---|---|---|
| 1 | 1163 | 93 | A2 " | yes | out-of-plane bending |
| 2 | 1213 | 14 | E' | very slight | in-plane bending |
| 3 | 1213 | 14 | E' | very slight | in-plane bending |
| 4 | 2582 | 0 | A1' | no | symmetric stretch |
| 5 | 2716 | 126 | E' | Yes | asymmetric stretch |
| 6 | 2716 | 126 | E' | Yes | asymmetric stretch |

By the 3N-6 rule, there should be 6 different vibration modes. However, there is only three peaks found in the IR spectrum. Only one peak is observed for the in-plane bending vibration as mode 2 and 3 are degenerated in energy. The same applies to the asymmetric stretch modes (mode 5 and 6), which are also degenerated in energy. There is no change in the dipole moment of the molecule for mode 3 (the symmetrical stretching vibration) and hence it doesn't lead to infrared absorption.
MO of BH3

Are there any significant differences between the real and LCAO MOs?
The real (calculated) MOs are generally more diffused than the LCAO (linear combination of atomic orbitals) MOs, especially for the unoccupied orbitals high in energy (from MO 5 to MO 8 as labelled in the above diagram). Hence, the real MO can look quite different from the LCAO prediction (e.g. MO 6 - the H3 FO is much larger than the LCAO has predicted; MO 8 - the electron density is spread across the whole molecule, unlike the prediction)
What does this say about the accuracy and usefulness of qualitative MO theory?
The qualitative MO theory are more accurate and useful for occupied MOs with low energy. Above the HOMO (highest occupied molecular orbital), the prediction are less accurate.
NH3
Method and Basis Set: B3LYP/6-31G(d,p)
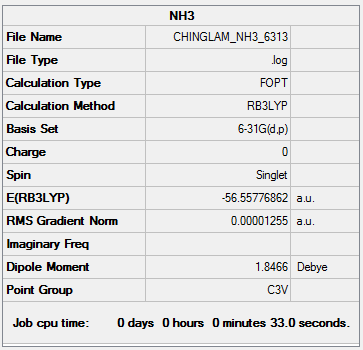
Optimization log file: CHINGLAM NH3 6313.LOG
Frequency analysis log file: CHINGLAM NH3 6313 FREQ.LOG
Item Table
Item Value Threshold Converged? Maximum Force 0.000017 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000064 0.001800 YES RMS Displacement 0.000028 0.001200 YES
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- -0.2239 -0.0404 -0.0040 7.1998 7.2613 27.9769 Low frequencies --- 1089.9671 1694.2126 1694.2129
Jmol Image
Ammonia |
NH3-BH3
Method and Basis Set: B3LYP/6-31G(d,p)
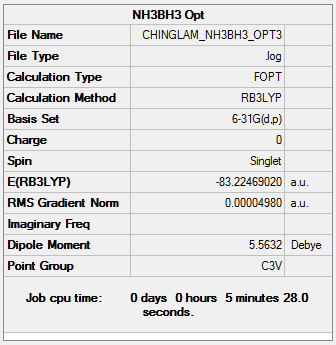
Optimization log file: CHINGLAM NH3BH3 OPT3.LOG
Frequency analysis log file: CHINGLAM NH3BH3 OPT3 FREQ2.LOG
Item Table
Item Value Threshold Converged? Maximum Force 0.000052 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.000423 0.001800 YES RMS Displacement 0.000145 0.001200 YES
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- -0.1703 -0.0781 -0.0066 12.6039 12.6146 12.8091 Low frequencies --- 263.1708 632.8934 638.8999
Jmol Image
Ammonia borane |
Association energies: Ammonia-Borane
From the above calculation (Method: B3LYP/6-31G(d,p)) :
E(NH3)= -56.55776862 a.u.
E(BH3)= -26.61532363 a.u.
E(NH3-BH3)= -83.22469020 a.u.
ΔE=E(NH3-BH3)-[E(NH3)+E(BH3)]
ΔE= (-83.22469020)- [(-56.55776862)+ (-26.61532363)] = -0.05159795 a.u. ≈ -0.05160 a.u. (5 d.p.)
ΔE= 2625.5 x -0.05159795 ≈ -135 kJ / mol
ΔE is negative, implying that the reaction is overall exothermic - the bond forming process is energy releasing. The association energy of the adduct is 135 kJ/mol. The means that the B-N dative bond is quite week relative to the stronger covalent bonds (For reference, some mean bond enthalpies data [2] are listed as following: C-H 420 kJ/mol, C-C 350 kJ/mol, O-H 463 kJ/mol). However, the enthalpy of B-N dative bond is quite similar to some of the weak covalent bond (O-O 146 kJ/mol, I-I 151 kJ/mol) and much higher than the enthalpy of hydrogen bonding (HO-H -- OH 2 22 kJ/mol).
Overall, B-N dative bond is consider to be weak comparing to covalent bonds, but it is much stronger than H-bonding and van der Waals interactions.
BBr3
Method and Basis Set: B3LYP/6-31G(d,p)LANL2DZ
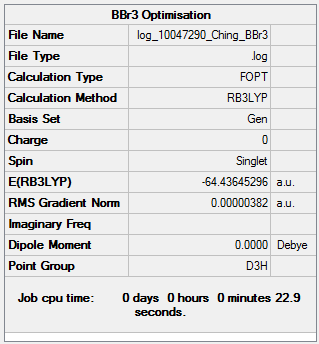
Optimization log file: Log 10047290 Ching BBr3.log
Frequency analysis log file: Log 10047294 Ching BBr3 freq.log
DSpace link: DOI:10042/202401
Item Table
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES Predicted change in Energy=-4.027119D-10 Optimization completed.
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Jmol Image
B3LYP/6-31G(d,p)LANL2DZ |
Smf115 (talk) 00:03, 26 May 2018 (BST)Overall, a very good first section with clear thought given to the answers throughout.
Project Section -- Lewis Acids and Bases - Main Group Halide
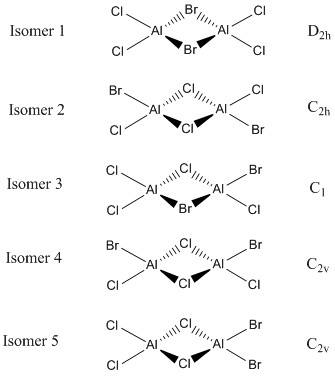
The Isomers of AlBr2Cl4:
AlBr2Cl4 (2 Bridging Br Ions) - Isomer 1
Method and Basis Set: B3LYP/6-31G(d,p)LANL2DZ
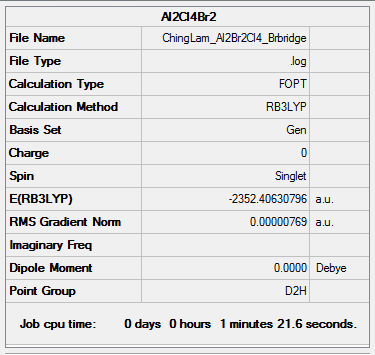
Optimization log file: ChingLam Al2Br2Cl4 Brbridge.log
Frequency analysis log file: ChingLam Al2Br2Cl4 Brbridge freq.log
DSpace link: DOI:10042/202404
Item Table
Item Value Threshold Converged? Maximum Force 0.000029 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000664 0.001800 YES RMS Displacement 0.000278 0.001200 YES Predicted change in Energy=-1.390401D-08 Optimization completed.
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- -5.1253 -5.0805 -3.1847 -0.0051 -0.0047 -0.0045 Low frequencies --- 14.8608 63.2610 86.0512
Jmol Image
Isomer 1 |
AlBr2Cl4 (The Isomer with Trans Terminal Br and Bridging Cl Ions) - Isomer 2
Method and Basis Set: B3LYP/6-31G(d,p)LANL2DZ
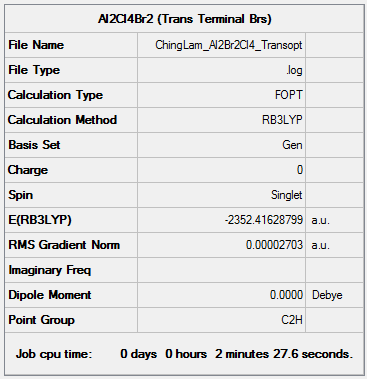
Optimization log file: ChingLam Al2Br2Cl4 Transopt.log
Frequency analysis log file: ChingLam Al2Br2Cl4 Transopt freq.log
DSpace link: DOI:10042/202406
Item Table
Item Value Threshold Converged? Maximum Force 0.000084 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.001800 0.001800 YES RMS Displacement 0.000622 0.001200 YES Predicted change in Energy=-1.168706D-07 Optimization completed.
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- -3.5004 -2.4150 0.0007 0.0025 0.0027 0.7437 Low frequencies --- 17.7463 49.0305 72.9456
Jmol Image
Isomer 2 |
Relative Energy of Isomer 1 and 2
E(Isomer 1)= -2352.40630796 a.u.
E(Isomer 2)= -2352.41628799 a.u.
Relative Energy = E(Isomer 1)- E(Isomer 2) = (-2352.40630796) - (-2352.41628799) = 0.00998002999 a.u.
Relative Energy in kJ/mol = 0.00998002999 x 2625.5 = 26 kJ/mol (Round to whole no.)
Discuss the relative stability of these conformers with respect to the bridging ions.
The above calculations demonstrate that isomer 2 is lower in energy comparing to isomer 1 by 26 kJ/mol. This implies that isomer 2 is more stable than isomer 1. The difference in the stability is due to the difference bridging ions of the conformer. Isomer 2 has two Cl ions as the bridging atoms, while isomer 1 has two bridging Br ions. Cl is in row 3 of the periodic table and Br is in row 4. As Cl and Al are in the same row in the periodic table, their valence orbitals would be similar in size and energy comparing to Br. In the case for isomer 2 in the bridging region of the molecule, there would be better overlaps between the fragment orbitals (FOs) and greater stabilization from smaller FOs energy difference comparing to isomer 1. Apart from the electronic perspectives, Cl ions are smaller than Br ions, which makes the Cl bridging more sterically favorable and contributes to the higher stability.
AlCl2Br (Monomer)
Method and Basis Set: B3LYP/6-31G(d,p)LANL2DZ
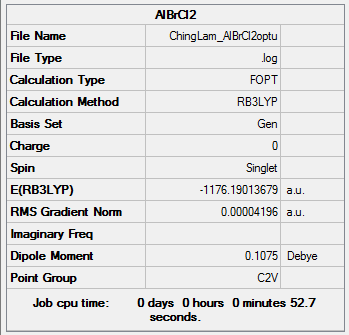
Optimization log file: ChingLam AlBrCl2optu.log
Frequency analysis log file: ChingLam AlBrCl2optu freq.log
DSpace link: DOI:10042/202409
Item Table
Item Value Threshold Converged? Maximum Force 0.000136 0.000450 YES RMS Force 0.000073 0.000300 YES Maximum Displacement 0.000681 0.001800 YES RMS Displacement 0.000497 0.001200 YES Predicted change in Energy=-7.984435D-08 Optimization completed.
Low Frequencies Lines from the Frequency Analysis Log File
Low frequencies --- 0.0010 0.0029 0.0037 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Jmol Image
Monomer |
Dissociation energy of Isomer 2 into 2AlCl2Br
2AlCl2Br --> Al2Cl4Br2
From the above calculation (Method: B3LYP/6-31G(d,p)LANL2DZ):
E(Al2Cl4Br2, Isomer 2)= -2352.41628799 a.u.
E(AlCl2Br) = -1176.19013679 a.u.
Association Energy = E(Al2Cl4Br2, Isomer 2) - 2E(AlCl2Br)
Dissociation Energy = - (Association Energy)
Dissociation Energy = 2E(AlCl2Br) - E(Al2Cl4Br2, Isomer 2) = 2(-1176.19013679) - (-2352.41628799) = 0.03601441 a. u.
Dissociation Energy in kJ/mol = 0.03601441 x 2625.5 = 95 kJ/mol (Round to whole no.)
The dissociation of the dimer into monomers is positive and endothermic as bonds are broken in the reaction. Bond breaking takes in energy.
Is the product more or less stable than the isolated monomers?
As Dissociation Energy = - (Association Energy), the association of the monomers into dimers is exothermic (association energy is negative) from the above calculation. This implies that the product (the Al2Cl4Br2 dimer) is more stable than the isolated monomers.
The AlCl2Br monomer is two electron short from a full octet. Hence, the Al centre of the isolated monomer is electron deficient. By forming dimer with lone pair electron donation from the bridging Cl, the electron deficiency at Al is relief with full octet fulfilled, making the system more stable overall.
Smf115 (talk) 13:01, 27 May 2018 (BST)Correct calculation with consideration towards the accuracy of the final reported energy. Great explaination to justify the result.
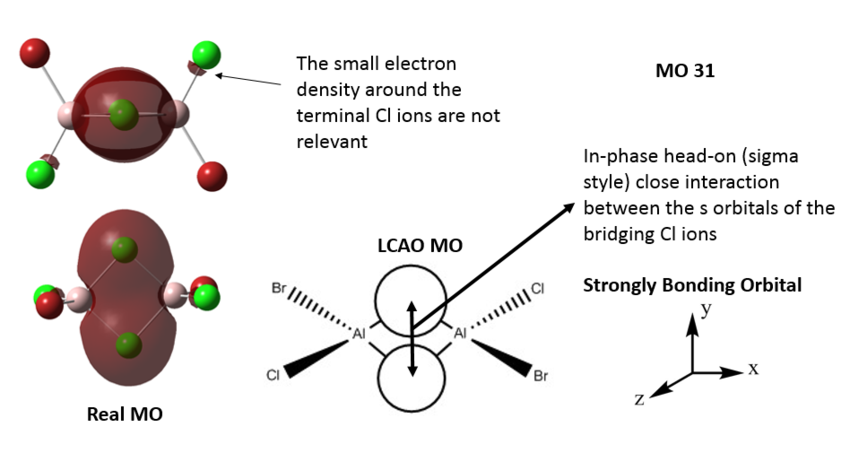
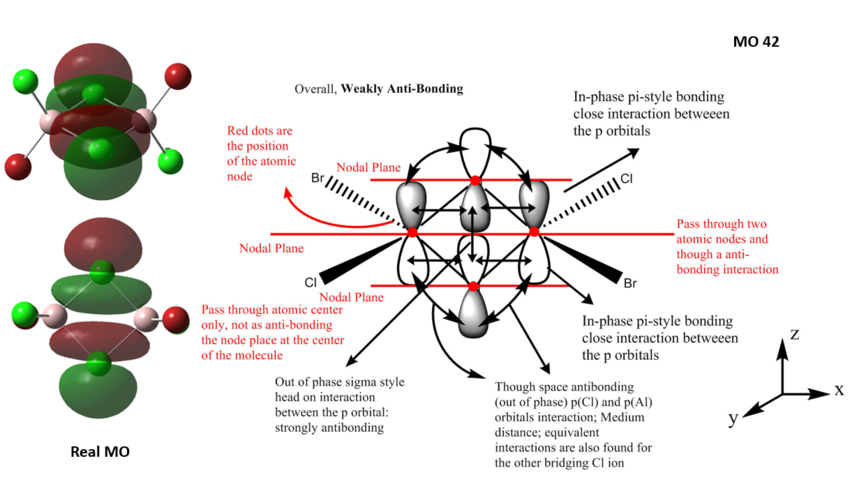
MO of Al2Cl4Br2, Isomer 2
All the occupied valence MOs of Al2Cl4Br2 (isomer 2) are visualised and 3 MOs ranging from highly bonding to highly antibonding are presented below with their LCAO MO diagrams. The interactions occurring in the MOs are annotated.
MO31 - highly bonding MO
MO42 - weakly anti-bonding MO
MO52- highly anti-bonding MO


Smf115 (talk) 13:08, 27 May 2018 (BST)Excellent project section with clearly presented MOs and thorough annotations of the interactions and nodal planes to justify the MO character overall.
Reference and Acknowledgement
Acknowledgement
The BH3 MO diagram in BH3 section of this report is constructed based on Fig. 5 in this online document [1].
