Rep:Mod:01106385
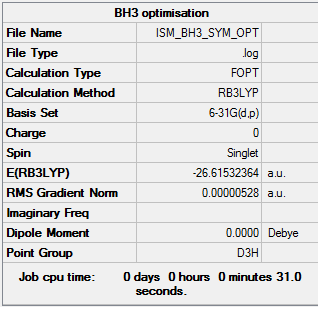
BH3
RB3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000042 0.001800 YES RMS Displacement 0.000027 0.001200 YES
Frequency file: ISM BH3 FREQ.LOG
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
Optimised BH3 molecule |
Vibrational analysis
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
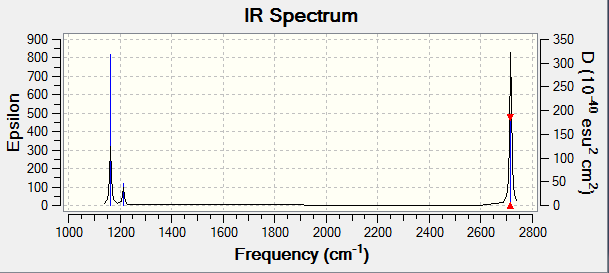
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E | yes | bend |
| 1213 | 14 | E | yes | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
There are 6 vibrations in this molecule as shown in the table but there are only 3 peaks as shown in the spectrum. This is because there are 2 sets of degenerate vibrations: 1213 and 2716 cm-1. These degenerate vibrations each give rise to a single peak in the spectrum and one of the vibrations is not IR active so there is no peak in the spectrum corresponding to this vibration. Therefore, there is only 3 peaks in the spectrum.
Molecular orbitals analysis

There are no significant differences between the real and LCAO molecualr orbitals (MOs) as can be seen in figure 1. This tells us that qualitative MO theory is quite accurate and useful to predict the shape of the real MOs of a molecule.
Ng611 (talk) 14:37, 31 May 2018 (BST) Good analysis. Are there any differences at all between qualitative MO theory and the calculated MOs?
Association energies: Ammonia-Borane
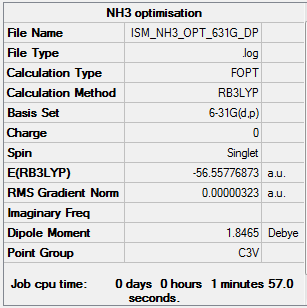
NH3
RB3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency file: ISM NH3 FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Optimised NH3 molecule |
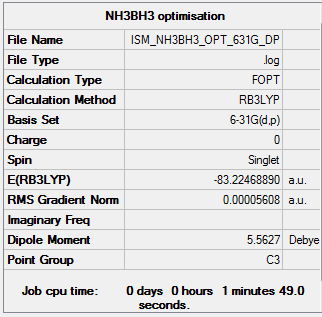
NH3BH3
RB3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.000903 0.001800 YES RMS Displacement 0.000344 0.001200 YES
Frequency file: ISM NH3BH3 FREQ.LOG
Low frequencies --- -27.8630 -0.2737 -0.0651 0.0891 11.5835 11.6805 Low frequencies --- 261.3510 631.2489 637.5432
Optimised NH3BH3 molecule |
Bond energy determination
- E(NH3)= -56.55777 a.u.
- E(BH3)= -26.61532 a.u.
- E(NH3BH3)= -83.22469 a.u.
Using the equation ΔE=E(NH3BH3)-[E(NH3)+E(BH3)], where ΔE is the association energy, and the above reported energies, the association energy of the B-N bond is (-83.22469)-[(-56.55777)+(-26.61532)] = -0.0516 a.u. = -135.47581 kJ/mol
Ng611 (talk) 14:38, 31 May 2018 (BST) correct answer but, the final value should be reported to the nearest kj/mol
This is a weak bond as the bond energy is similar to the bond energy of an O-O bond (146 kJ/mol) which is a weak bond and is a lot less than the bond energy of a strong bond such as H-F (565 kJ/mol).[2]
Using Pseudo-potentials and different basis sets
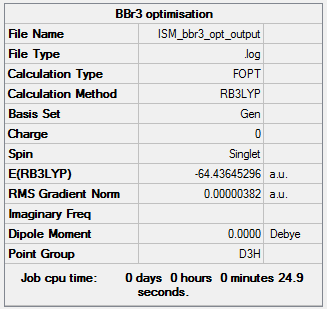
BBr3
RB3LYP/LanL2DZ
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency file: ISM bbr3 freq output.log
Frequency file on DSpace: DOI:10042/202410
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Optimised BBr3 molecule |
Ionic Liquids project: Designer Solvents
[N(CH3)4]+
RB3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000767 0.001800 YES RMS Displacement 0.000249 0.001200 YES
Frequency file: N FREQ ISM.LOG
Low frequencies --- -22.2614 -14.9273 -11.4472 -0.0010 -0.0007 0.0002 Low frequencies --- 180.5332 281.8698 286.4332
Optimised [N(CH3)4]+ molecule |
MO analysis
Using Gaussview, the molecular orbitals were calculated and three of them are presented in the following sections.
MO 6
The LCAO for this orbital is shown in figure 2 and the calculated MO is shown in figure 3. For this orbital the s orbital on the nitrogen is in phase with the ligand orbitals, which are represented as s orbitals here, giving a bonding orbital with an energy of -1.19646 a.u.


MO 9
The LCAO for this orbital is shown in figure 4 and the calculated MO is shown in figure 5. For this orbital the contribution from the nitrogen atom is the pz orbital. The ligand orbitals, which are represented as s orbitals here, have differeing phases. This gives a bonding orbital with an energy of -0.92555 a.u.


Ng611 (talk) 14:53, 31 May 2018 (BST) For your second FO, I don't see any nodes in the methyl groups of the calculated orbital.
MO 21
The LCAO for this orbital is shown in figure 6 and the calculated MO is shown in figure 7. For this orbital the contribution from the nitrogen atom is the pz orbital. The ligand orbitals are represented as px orbitals here. This gives an anti-bonding orbital with an energy of -0.57934 a.u. This is the highest occupied MO, giving it the title of HOMO.


Ng611 (talk) 14:50, 31 May 2018 (BST) Close but not quite for your FO analysis here. You can see that one hydrogen in each methyl group has no orbital contribution. Thus your FO diagram as drawn for this one is incorrect as it predicts that you have significant FO involvement from all three hydrogens.
[P(CH3)4]+
RB3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000134 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000771 0.001800 YES RMS Displacement 0.000271 0.001200 YES
Frequency file: P FREQ ISM.LOG
Low frequencies --- -19.3897 -18.3624 -0.0029 -0.0022 -0.0019 42.4113 Low frequencies --- 160.3810 191.0350 191.3916
Optimised [P(CH3)4]+ molecule |
NBO charge analysis


As can be seen in figures 8 and 9 the charge distribution in the two cations vary quite a lot:
- [N(CH3)4]+:
- Central nitrogen atom: -0.295
- Carbon atoms: -0.483
- Hydrogen atoms: 0.269
- [P(CH3)4]+:
- Central phosphorus atom: 1.666
- Carbon atoms: -1.060
- Hydrogen atoms: 0.298
The main difference here is that the central atom in the nitrogen compound has a negative charge whereas the central atom in the phosphorus compound has a positive charge. This is because C has a higher electronegativity value (2.55) than phosphorus (2.19) but lower than the value for nitrogen (3.04). This leads to the N-C bond being polar with the N atom having the electron density on it so it has a negative charge. This is opposite to the P-C bond where the electron density is on the C atom and therefore, the P atom has a positive charge. This is also the basis of the reason that the C atoms have a more negative charge in the P compound than they do in the N compound as the C atom gets the electron density from the C-H bond (H electronegativity: 2.20) in both compounds, therefore, this electron density combines with the electron density from the P-N bond and the charge is more negative than it is in the N compound where this doesn't happen and is only negative due to the delta negative charge from the C-H bond.[3]
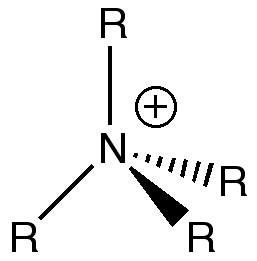
As can been in figure 10, a positive charge is often placed on the N atom in a depiction of [NR4]+, this charge represents the charge of the overall compound being +1 and the fact that there is a lone pair of electrons on the nitrogen atom donating to the fourth methyl group as a nitrogen atom usually only has 3 substituents, but as can be seen in figure 8, the actual charge on the N atom isn't positive, rather it is negative. The positive charge is actually located on hydrogen atoms as also shown in figure 8.
Ng611 (talk) 14:46, 31 May 2018 (BST) Good charge analysis. I would also discuss some other features. For example: what is the summation of the partial charges (it may seem obvious, but it should be mentioned), are the charges the same for symmetrically related atoms, how different are the charges on the hydrogen atoms given the electronegativity differences of the two species?
References
- ↑ Hunt, P. (n.d.). Lecture 4 Tutorial - MO diagram for BH3. [ebook] p.3. Available at: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf.
- ↑ Petrucci, R., Herring, F., Madura, J. and Bissonnette, C. (n.d.). General chemistry, 2015.
- ↑ Helmenstine, T. (2018). List of Electronegativity Values of the Elements. [online] Science Notes and Projects. Available at: https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/ [Accessed 16 May 2018].
- ↑ Hunt, P. (n.d.). Computational Inorganic Chemistry: Hunt Research Goup, Imperial College London. [online] Huntresearchgroup.org.uk. Available at: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/12c_ionic_liquids.html [Accessed 16 May 2018].
Ng611 (talk) 14:54, 31 May 2018 (BST) Overall, a good report. Take care with regards to your LCAO analysis -- remember that not every atom has to contribute to every orbital all the time. Otherwise, a very good report -- well done.