Rep:Mod:00636773MP2
Introduction
In this project, the protonations of pnictogen heteroaromatic systems were computed to show how the position of a second heteroatom affects the molecule. The dipole moments, spectra and charge distribution were also computed. The following protonations of nitrogen and phosphorus molecules were considered:
Calculations
The following calculations were performed. 1,4-diphosphinine is highlighted for having a negative frequency, -126.80 cm-1.
| Property: | Pyridine: | Pyridinium: | |||||
|---|---|---|---|---|---|---|---|
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -248.29259330 a.u. | -248.29259330 a.u. | -248.29259330 a.u. | -248.66806092 a.u. | -248.66806092 a.u. | -248.66806092 a.u. | |
| Gradient: | 0.00000346 a.u. | 0.00000354 a.u. | 0.00000131 a.u. | 0.00000143 a.u. | |||
| Dipole moment: | 2.1842 Debye | 2.1842 Debye | 2.1842 Debye | 1.8723 Debye | 1.8723 Debye | 1.8723 Debye | |
| Point group: | C1 | C1 | C1 | C1 | C1 | C1 | |
| Calculation time: | 5:43.4 | 8:45.0 | 1:15.0 | 7:19.9 | 10:3.7 | 1:22.1 | |
| Link to D-Space: | DOI:10042/25524 | DOI:10042/25532 | DOI:10042/25540 | DOI:10042/25507 | DOI:10042/25516 | DOI:10042/25548 | |
| Property: | 1,2-diazine: | 1,2-diazinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -264.29361932 a.u. | -264.29361932 a.u. | -264.29361932 a.u. | -264.66250320 a.u. | -264.66250320 a.u. | -264.66250320 a.u. | |
| Gradient: | 0.00000313 a.u. | 0.00000314 a.u. | 0.00000377 a.u. | 0.00000370 a.u. | |||
| Dipole moment: | 4.1040 Debye | 4.1040 Debye | 4.1040 Debye | 2.2958 Debye | 2.2957 Debye | 2.2957 Debye | |
| Point group: | C1 | C1 | C1 | C1 | C1 | C1 | |
| Calculation time: | 7:51.5 | 7:7.6 | 1:3.4 | 9:0.7 | 8:45.4 | 1:20.1 | |
| Link to D-Space: | DOI:10042/25530 | DOI:10042/25539 | DOI:10042/25541 | DOI:10042/25508 | DOI:10042/25523 | DOI:10042/25549 | |
| Property: | 1,3-diazine: | 1,3-diazinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -264.32948401 a.u. | -264.32948401 a.u. | -264.32948401 a.u. | -264.68700624 a.u. | -264.68700624 a.u. | -264.68700624 a.u. | |
| Gradient: | 0.00000132 a.u. | 0.00000138 a.u. | 0.00000173 a.u. | 0.00000168 a.u. | |||
| Dipole moment: | 2.2867 Debye | 2.2867 Debye | 2.2867 Debye | 3.6769 Debye | 3.6769 Debye | 3.6769 Debye | |
| Point group: | C1 | C1 | C1 | C1 | C1 | C1 | |
| Calculation time: | 4:4.3 | 7:15.2 | 1:7.7 | 8:17.5 | 7:52.9 | 1:20.8 | |
| Link to D-Space: | DOI:10042/25525 | DOI:10042/25533 | DOI:10042/25542 | DOI:10042/25509 | DOI:10042/25517 | DOI:10042/25542 | |
| Property: | 1,4-diazine: | 1,4-diazinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -264.32301058 a.u. | -264.32301058 a.u. | -264.32301058 a.u. | -264.67641011 a.u. | -264.67641011 a.u. | -264.67641011 a.u. | |
| Gradient: | 0.00000459 a.u. | 0.00000465 a.u. | 0.00000559 a.u. | 0.00000570 a.u. | |||
| Dipole moment: | 0.0000 Debye | 0.0000 Debye | 0.0000 Debye | 4.4239 Debye | 4.4239 Debye | 4.4239 Debye | |
| Point group: | C1 | C1 | C1 | CS | CS | CS | |
| Calculation time: | 6:27.5 | 7:14.0 | 1:6.0 | 7:17.5 | 7:43.6 | 1:10.4 | |
| Link to D-Space: | DOI:10042/25526 | DOI:10042/25534 | DOI:10042/25543 | DOI:10042/25510 | DOI:10042/25518 | DOI:10042/25551 | |
| Property: | Phosphorine: | Phosphorinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -534.88604051 a.u. | -534.88604051 a.u. | -534.88604051 a.u. | -535.20989682 a.u. | -535.20989682 a.u. | -535.20989682 a.u. | |
| Gradient: | 0.00000381 a.u. | 0.00000375 a.u. | 0.00000293 a.u. | 0.00000283 a.u. | |||
| Dipole moment: | 1.8271 Debye | 1.8271 Debye | 1.8271 Debye | 1.5946 Debye | 1.5946 Debye | 1.5946 Debye | |
| Point group: | C1 | C1 | C1 | C1 | C1 | C1 | |
| Calculation time: | 11:43.4 | 9:3.5 | 1:18.6 | 12:34.8 | 10:13.9 | 1:25.5 | |
| Link to D-Space: | DOI:10042/25527 | DOI:10042/25535 | DOI:10042/25544 | DOI:10042/25511 | DOI:10042/25519 | DOI:10042/25552 | |
| Property: | 1,2-diphosphinine: | 1,2-diphosphinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -837.52677035 a.u. | -837.52677035 a.u. | -837.52677035 a.u. | -837.85352415 a.u. | -837.85352415 a.u. | -837.85352415 a.u. | |
| Gradient: | 0.00000242 a.u. | 0.00000231 a.u. | 0.00000173 a.u. | 0.00000178 a.u. | |||
| Dipole moment: | 3.0030 Debye | 3.0030 Debye | 3.0030 Debye | 1.6975 Debye | 1.6975 Debye | 1.6975 Debye | |
| Point group: | C1 | C1 | C1 | C1 | C1 | C1 | |
| Calculation time: | 26.56.8 | 7:57.8 | 1:13.9 | 29:59.5 | 9:25.0 | 1:28.4 | |
| Link to D-Space: | DOI:10042/25531 | DOI:10042/25538 | DOI:10042/25546 | DOI:10042/25512 | DOI:10042/25520 | DOI:10042/25553 | |
| Property: | 1,3-diphosphinine: | 1,3-diphosphinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -837.51276456 a.u. | -837.51276456 a.u. | -837.51276456 a.u. | -837.83435156 a.u. | -837.83435156 a.u. | -837.83435156 a.u. | |
| Gradient: | 0.00000513 a.u. | 0.00000525 a.u. | 0.00000233 a.u. | 0.00000234 a.u. | |||
| Dipole moment: | 1.5646 Debye | 1.5646 Debye | 1.5646 Debye | 3.2506 Debye | 3.2506 Debye | 3.2506 Debye | |
| Point group: | C1 | C1 | C1 | C1 | CS | CS | |
| Calculation time: | 11:52.0 | 7:57.9 | 1:15.2 | 22:22.9 | 8:23.9 | 1:25.0 | |
| Link to D-Space: | DOI:10042/25528 | DOI:10042/25536 | DOI:10042/25545 | DOI:10042/25513 | DOI:10042/25521 | DOI:10042/25555 | |
| Property: | 1,4-diphosphinine: | 1,4-diphosphinium: | |||||
| Optimisation | Frequency | NBO | Optimisation | Frequency | NBO | ||
| File type: | .log | .log | .log | .log | .log | .log | |
| Calculation type: | FOPT | Freq | SP | FOPT | Freq | SP | |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | RB3LYP | |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | |
| Final energy: | -837.51008246 a.u. | -837.51008246 a.u. | -837.51008246 a.u. | -837.82920862 a.u. | -837.82920862 a.u. | -837.82920862 a.u. | |
| Gradient: | 0.00000464 a.u. | 0.00000462 a.u. | 0.00000136 a.u. | 0.00000140 a.u. | |||
| Dipole moment: | 0.0000 Debye | 0.0000 Debye | 0.0000 Debye | 3.7474 Debye | 3.7474 Debye | 3.7474 Debye | |
| Point group: | C1 | C1 | C1 | C1 | C1 | C1 | |
| Calculation time: | 17:49.3 | 7:47.2 | 1:7.9 | 22:14.1 | 9:20.7 | 1:19.3 | |
| Link to D-Space: | DOI:10042/25529 | DOI:10042/25537 | DOI:10042/25547 | DOI:10042/25514 | DOI:10042/25522 | DOI:10042/25554 | |
The convergence results can be found here.
Additional Calculations
Corrected 1,4-diphosphorinium
The symmetry of the original file was broken manually and the ion was reoptimised as follows:
| Property: | 1,4-diphosphorinium: | ||
|---|---|---|---|
| Optimisation | Frequency | NBO | |
| File type: | .log | .log | .log |
| Calculation type: | FOPT | Freq | SP |
| Calculation method: | RB3LYP | RB3LYP | RB3LYP |
| Basis set: | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) |
| Final energy: | -837.82946366 a.u. | -837.82946366 a.u. | -837.82946366 a.u. |
| Gradient: | 0.00000077 a.u. | 0.00000078 a.u. | |
| Dipole moment: | 3.4625 Debye | 3.4625 Debye | 3.4625 Debye |
| Point group: | C1 | C1 | C1 |
| Calculation time: | 4:44.0 | 2:33.0 | 0:24.0 |
The convergence results can be found here.
Results and Analysis
Comparison of Dipole Moments of Protonated Heteroatoms
Below is a table of bond angles, bond lengths, dipole moments and NBO information for all of the molecules and their respective adducts with a proton. A central column is given that show the difference between the two. Bond angles are given anticlockwise from the protonated heteroatom. Note the dipole moments are given as scalars. The bond angles and bond lengths of benzene are 120° and 1.40Å respectively [1].
Energies are given as enthalpies of protonation in the gaseous phase.

| Name: | Pyridine: | Pyridinium: | ||
|---|---|---|---|---|
| NBO Analysis: | 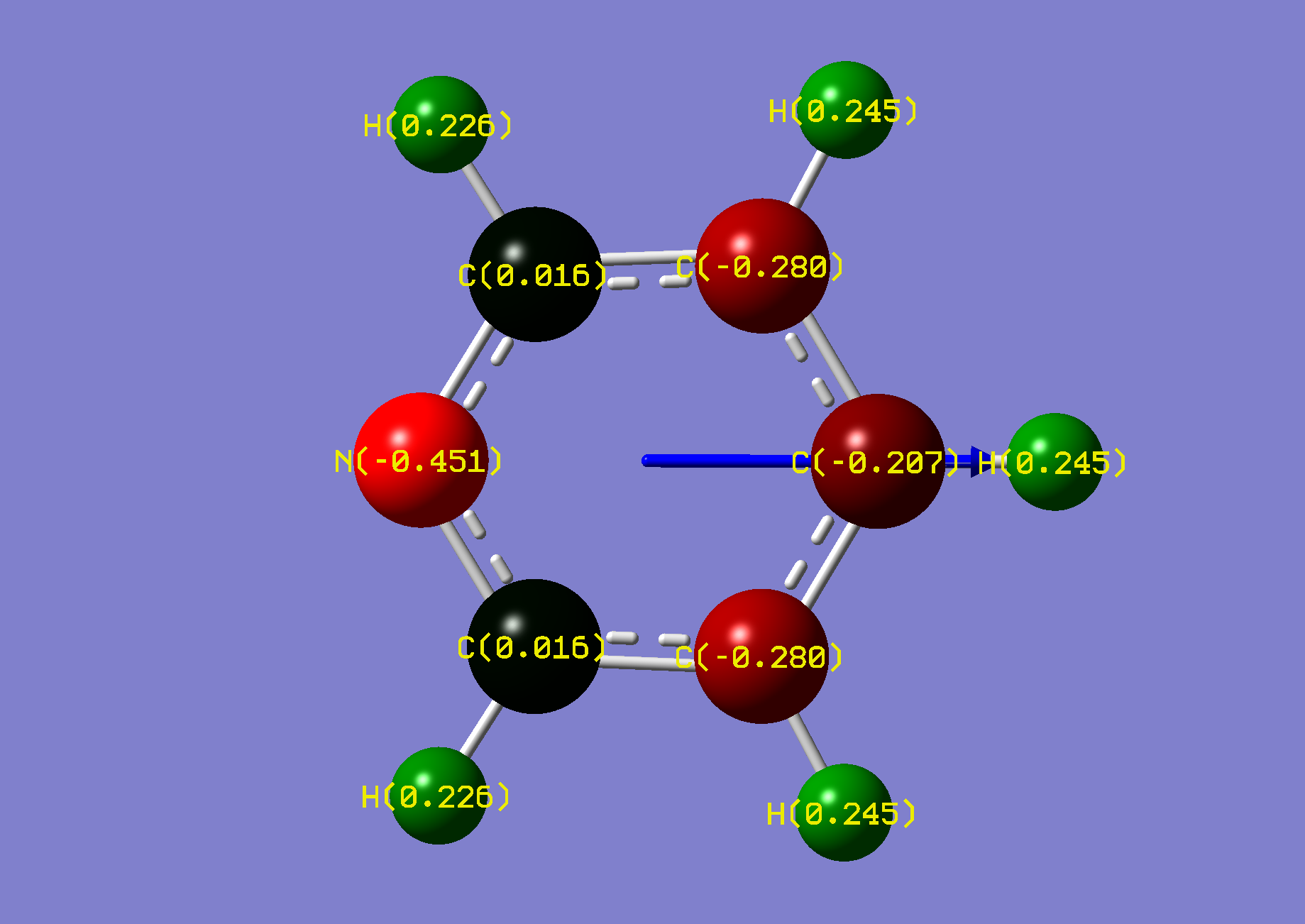 |
 | ||
| Dipole Moment: | 2.1842 (Lit. 2.22 [2]) Debye | -0.3119 Debye | 1.8723 Debye | |
| Bond angle 6-1-2: | 117.07° | +6.44° | 123.51° | |
| Bond angle 1-2-3: | 123.77° | -4.53° | 119.24° | |
| Bond angle 2-3-4: | 118.43° | +0.65° | 119.08 | |
| Bond angle 3-4-5: | 118.54° | +1.52° | 120.06° | |
| Bond angle 4-5-6: | 118.43° | +0.65° | 119.08° | |
| Bond angle 5-6-1: | 123.77° | -4.53° | 119.24° | |
| Bond length 1-2: | 1.34Å | +0.01Å | 1.35Å | |
| Bond length 2-3: | 1.40Å | -0.02Å | 1.38Å | |
| Bond length 3-4: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 4-5: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 5-6: | 1.40Å | -0.02Å | 1.38Å | |
| Bond length 6-1: | 1.34Å | +0.01Å | 1.35Å | |
| Energy/kJ·mol-1: | -651,892.25 | -985.79 | -652,878.04 | |
| Comments: | The dipole moment is flipped and reduced. Electron density is drawn from the ring in the pyridinium ion.
The angles and bond lengths tend towards that of benzene. | |||
| Name: | 1,2-Diazine: | 1,2-Diazinium: | ||
| NBO Analysis: | 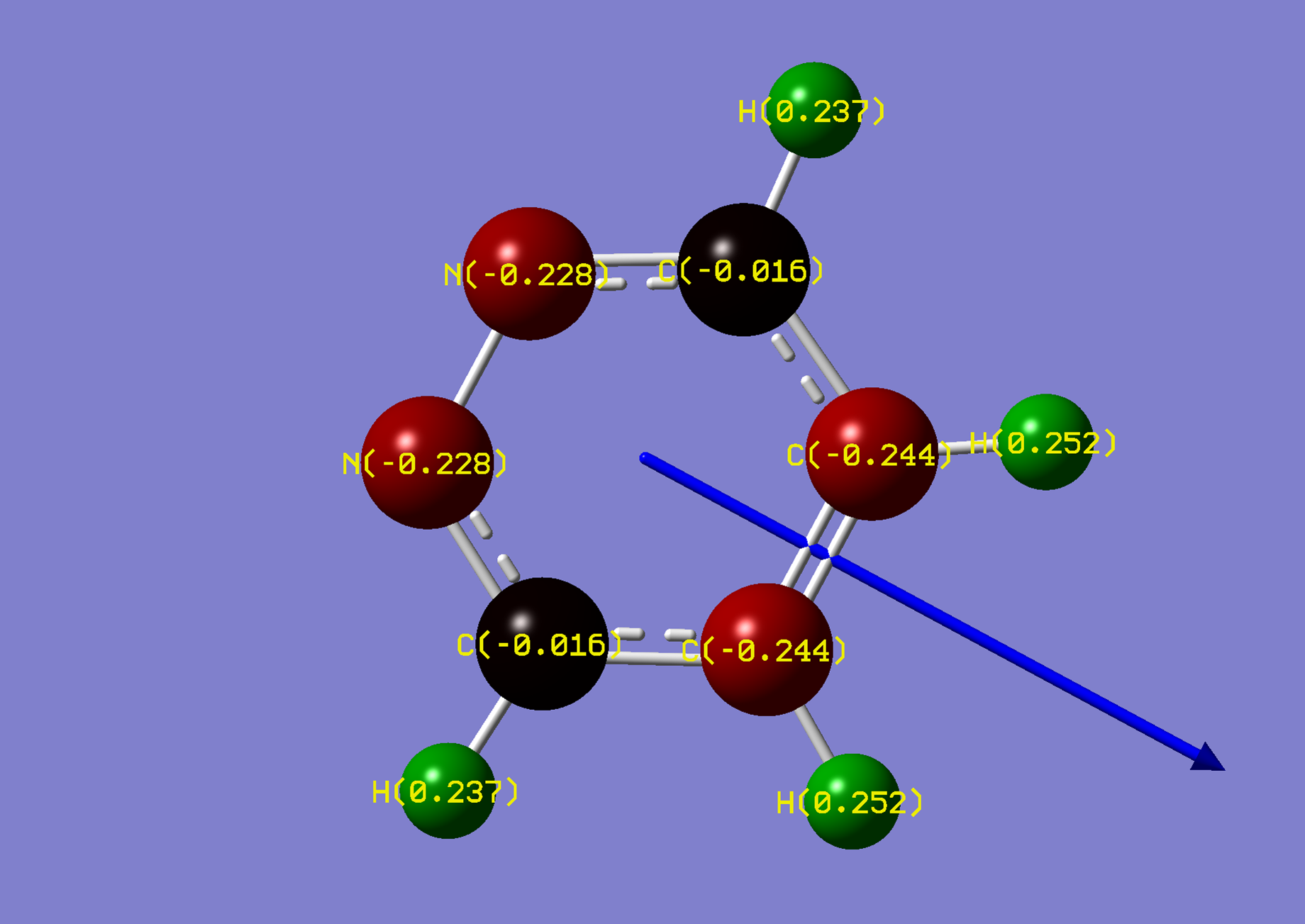 |
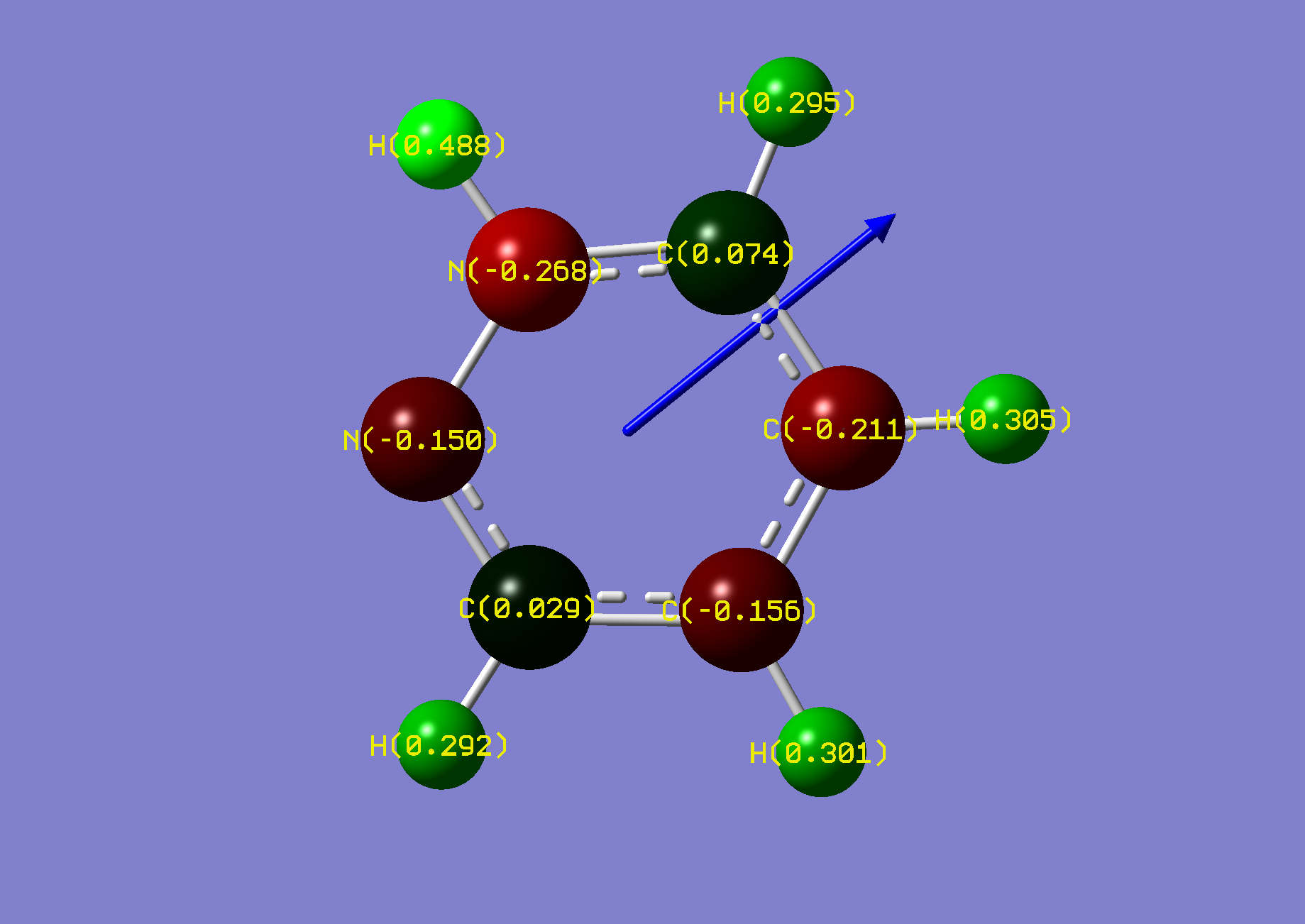 | ||
| Dipole Moment: | 4.1040 (Lit. 4.22 [2]) Debye | -1.8082 Debye | 2.2958 Debye | |
| Bond angle 6-1-2: | 119.36° | +7.43° | 126.70° | |
| Bond angle 1-2-3: | 119.36° | -3.65° | 115.71° | |
| Bond angle 2-3-4: | 123.81° | -0.67° | 123.14° | |
| Bond angle 3-4-5: | 116.83° | +1.73° | 118.56° | |
| Bond angle 4-5-6: | 116.83° | +0.64° | 117.47° | |
| Bond angle 5-6-1: | 123.81° | -5.39° | 118.42° | |
| Bond length 1-2: | 1.34Å | -0.01Å | 1.33Å | |
| Bond length 2-3: | 1.34Å | -0.01Å | 1.33Å | |
| Bond length 3-4: | 1.40Å | +0.01Å | 1.41Å | |
| Bond length 4-5: | 1.38Å | +0.01Å | 1.39Å | |
| Bond length 5-6: | 1.40Å | 0Å | 1.40Å | |
| Bond length 6-1: | 1.34Å | 0Å | 1.34Å | |
| Energy/kJ·mol-1: | -693,902.95 | -968.50 | -694,871.46 | |
| Comments: | The dipole moment is reduced and rotated towards the acidic proton. Some symmetry is lost, along with degeneracy. | |||
| Name: | 1,3-Diazine: | 1,3-Diazinium: | ||
| NBO Analysis: | 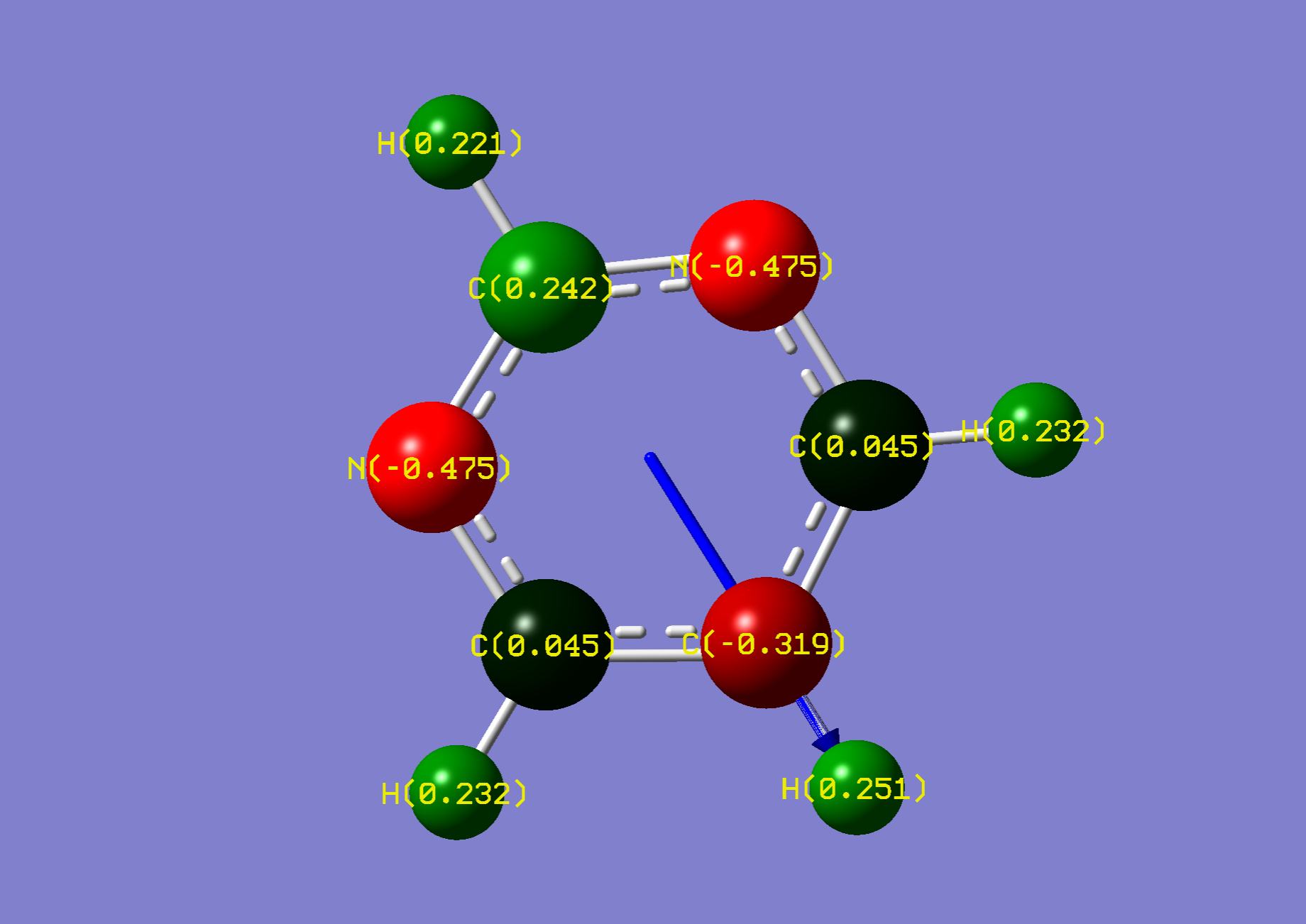 |
 | ||
| Dipole Moment: | 2.2867 (Lit. 2.33 [2]) Debye | +1.3902 Debye | 3.6769 Debye | |
| Bond angle 6-1-2: | 115.70° | +5.85° | 121.55° | |
| Bond angle 1-2-3: | 127.35° | -5.00° | 122.35° | |
| Bond angle 2-3-4: | 115.70° | +2.02° | 117.72° | |
| Bond angle 3-4-5: | 122.40° | +0.47° | 122.87° | |
| Bond angle 4-5-6: | 116.45° | +0.95° | 117.40° | |
| Bond angle 5-6-1: | 122.40° | -4.29° | 118.11° | |
| Bond length 1-2: | 1.34Å | +0.03Å | 1.37Å | |
| Bond length 2-3: | 1.34Å | -0.03Å | 1.31Å | |
| Bond length 3-4: | 1.34Å | 0Å | 1.34Å | |
| Bond length 4-5: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 5-6: | 1.39Å | -0.01Å | 1.38Å | |
| Bond length 6-1: | 1.34Å | +0.01Å | 1.35Å | |
| Energy/kJ·mol-1: | -693,997.11 | -938.67 | -694,935,79 | |
| Comments: | The dipole moment is increased and rotated towards the acidic proton.
Some symmetry is lost, but the bond angles tend towards benzene. | |||
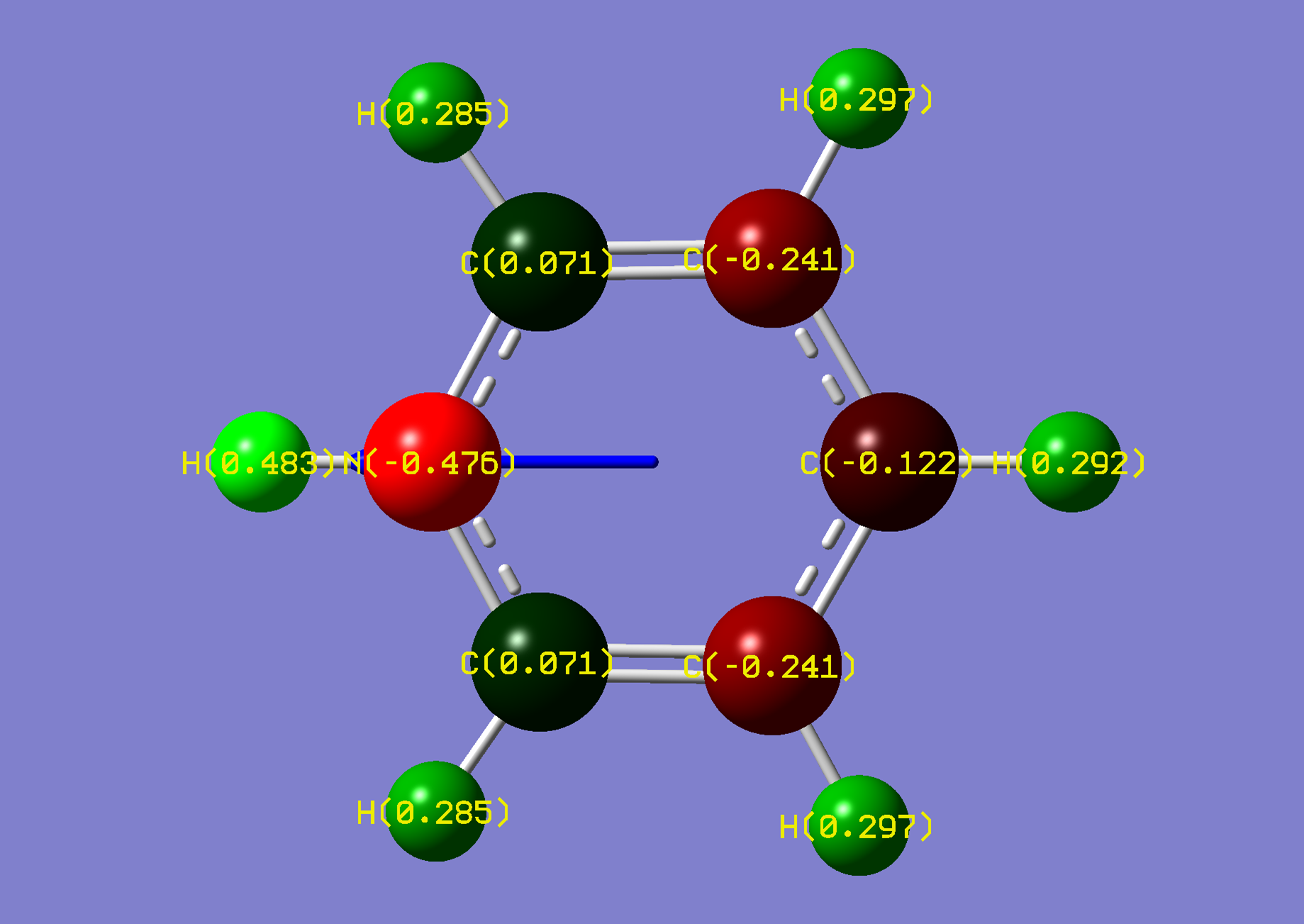
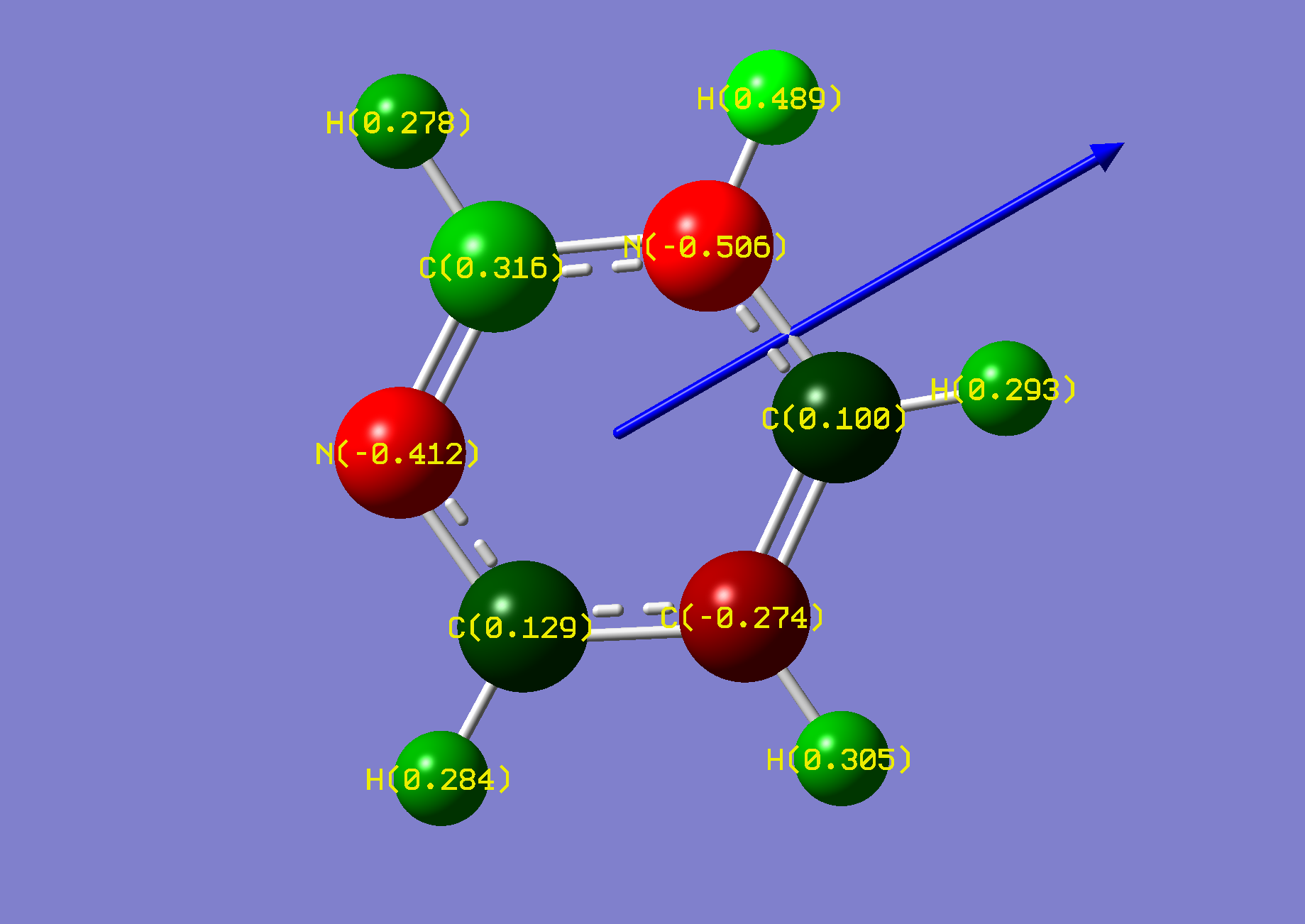
| Name: | 1,4-Diazine: | 1,4-Diazinium: | ||
| NBO Analysis: | 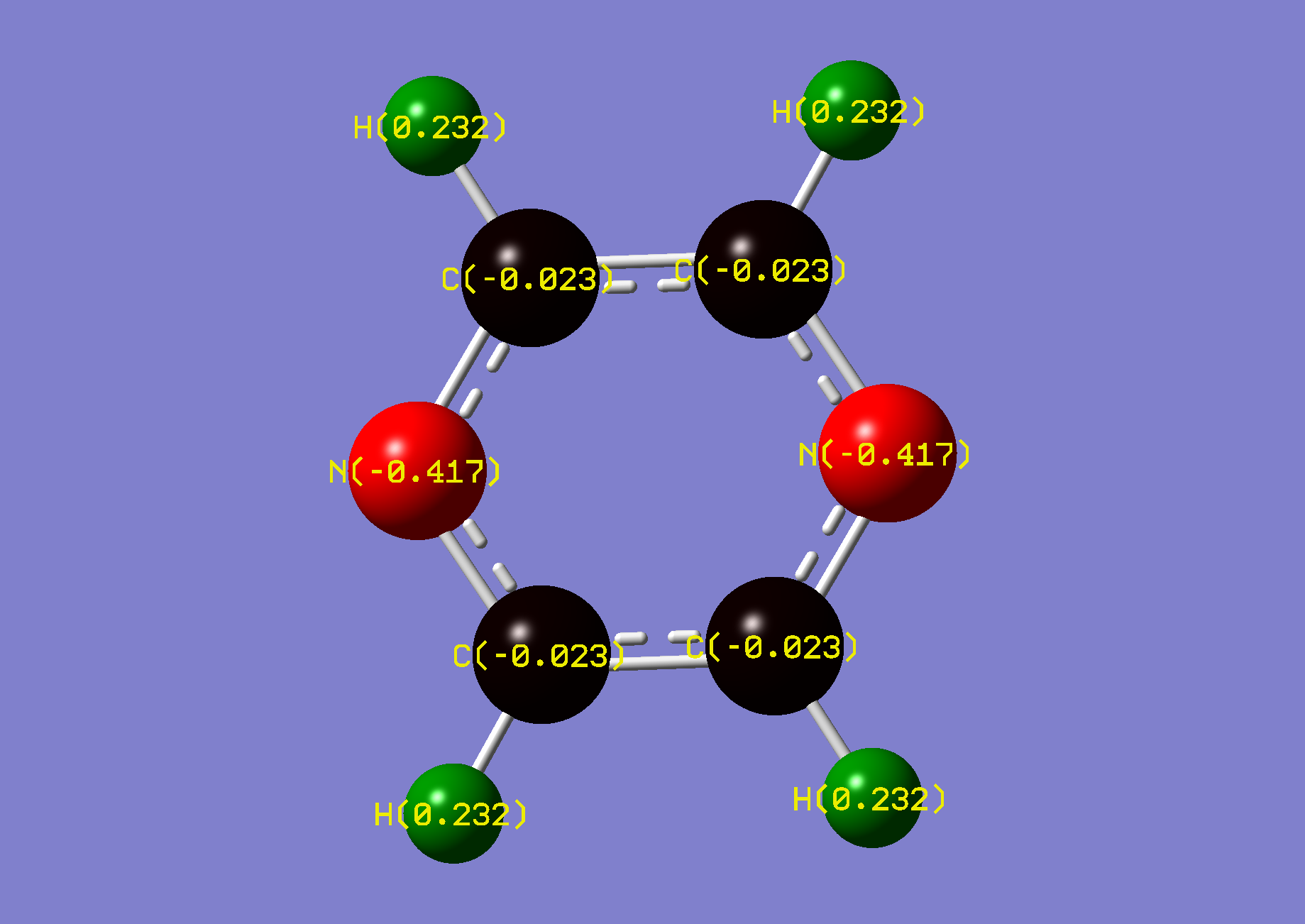 |
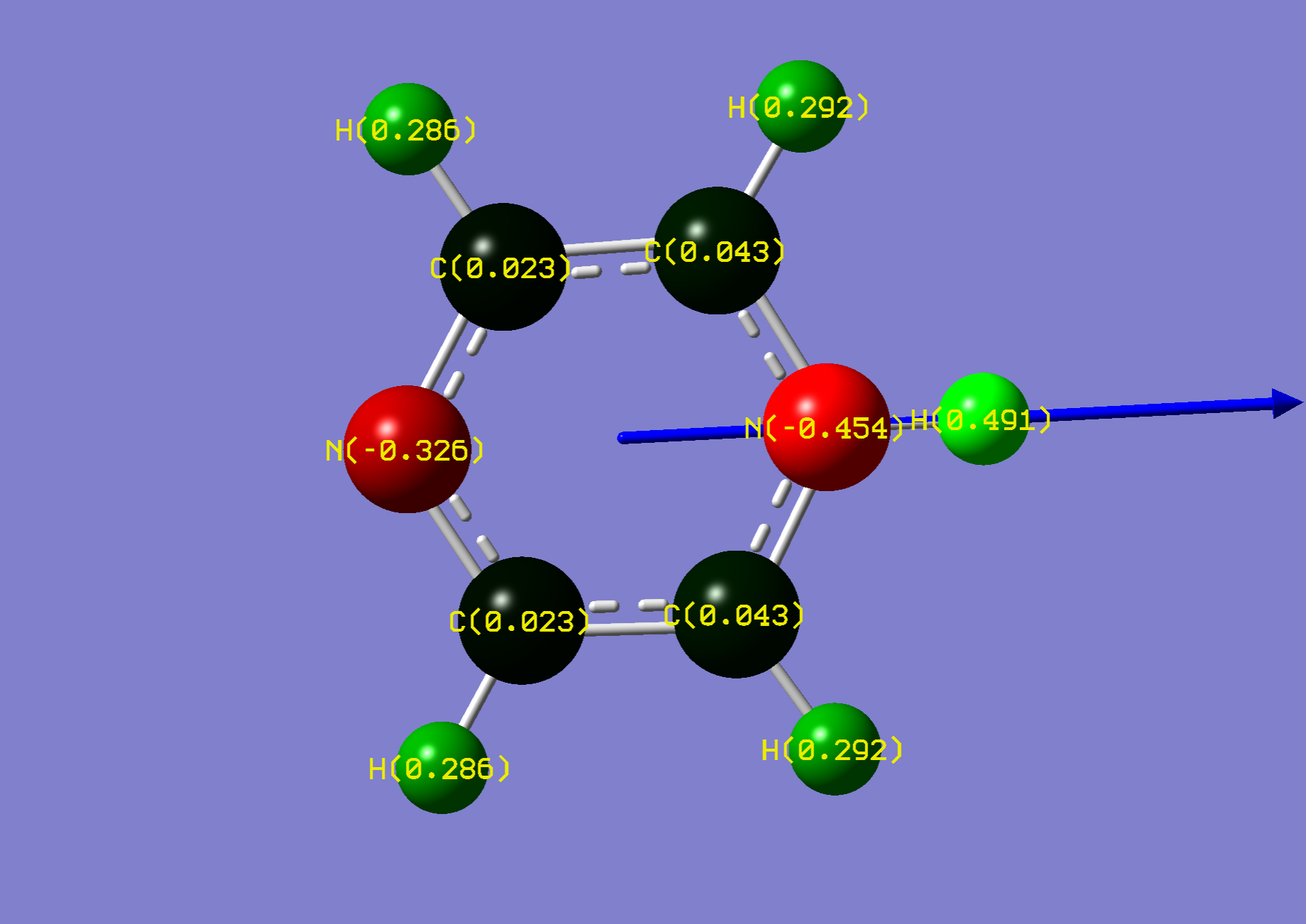 | ||
| Dipole Moment: | 0.0000 (Lit. 0.00 [2]) Debye | +4.4239 Debye | 4.4239 Debye | |
| Bond angle 6-1-2: | 115.79° | -3.42° | 112.37° | |
| Bond angle 1-2-3: | 122.11° | -4.63° | 117.48° | |
| Bond angle 2-3-4: | 122.11° | 0° | 122.11° | |
| Bond angle 3-4-5: | 115.79° | +2.65° | 118.44° | |
| Bond angle 4-5-6: | 122.11° | 0° | 122.11° | |
| Bond angle 5-6-1: | 122.11° | -4.63° | 117.48° | |
| Bond length 1-2: | 1.34Å | +0.01Å | 1.35Å | |
| Bond length 2-3: | 1.40Å | -0.01Å | 1.39Å | |
| Bond length 3-4: | 1.34Å | 0Å | 1.34Å | |
| Bond length 4-5: | 1.34Å | 0Å | 1.34Å | |
| Bond length 5-6: | 1.40Å | -0.01Å | 1.39Å | |
| Bond length 6-1: | 1.34Å | 0Å | 1.34Å | |
| Energy/kJ·mol-1: | -693,980.12 | -927.85 | -694,907.97 | |
| Comments: | A dipole moment is induced, suggesting an increase of solubility in aqueous acidic environments. Symmetry is lost upon protonation. | |||
| Name: | Phosphinine: | Phosphininium: | ||
| NBO Analysis: | 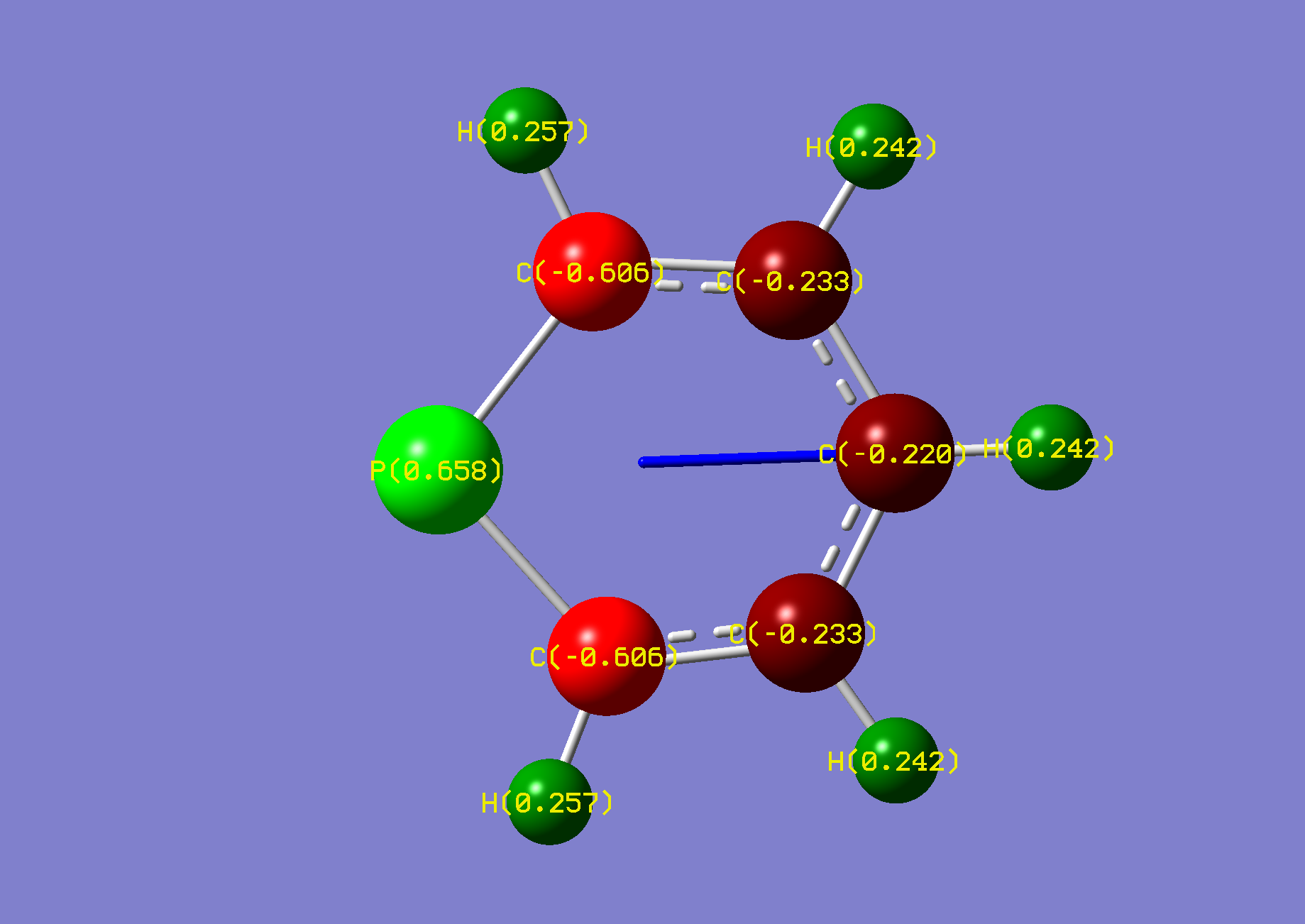 |
 | ||
| Dipole Moment: | 1.8271 Debye | -0.2325 Debye | 1.5946 Debye | |
| Bond angle 6-1-2: | 100.02° | +12.2° | 112.22° | |
| Bond angle 1-2-3: | 125.40° | -8.43° | 116.97° | |
| Bond angle 2-3-4: | 123.20° | +1.21° | 124.41° | |
| Bond angle 3-4-5: | 122.78° | 2.23° | 125.01° | |
| Bond angle 4-5-6: | 123.20° | 1.21° | 124.41° | |
| Bond angle 5-6-1: | 125.40° | -8.43° | 116.97° | |
| Bond length 1-2: | 1.75Å | -0.05Å | 1.70Å | |
| Bond length 2-3: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 3-4: | 1.40Å | 0Å | 1.40Å | |
| Bond length 4-5: | 1.40Å | 0Å | 1.40Å | |
| Bond length 5-6: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 6-1: | 1.75Å | -0.05Å | 1.70Å | |
| Energy/kJ·mol-1: | -1,404,343.41 | -850.28 | -1,405,193.69 | |
| Comments: | The highly strained phosphorus is relieved upon protonation.
Its bond length with the neighbouring carbons is reduced by 0.05Å. The dipole moment is flipped. | |||
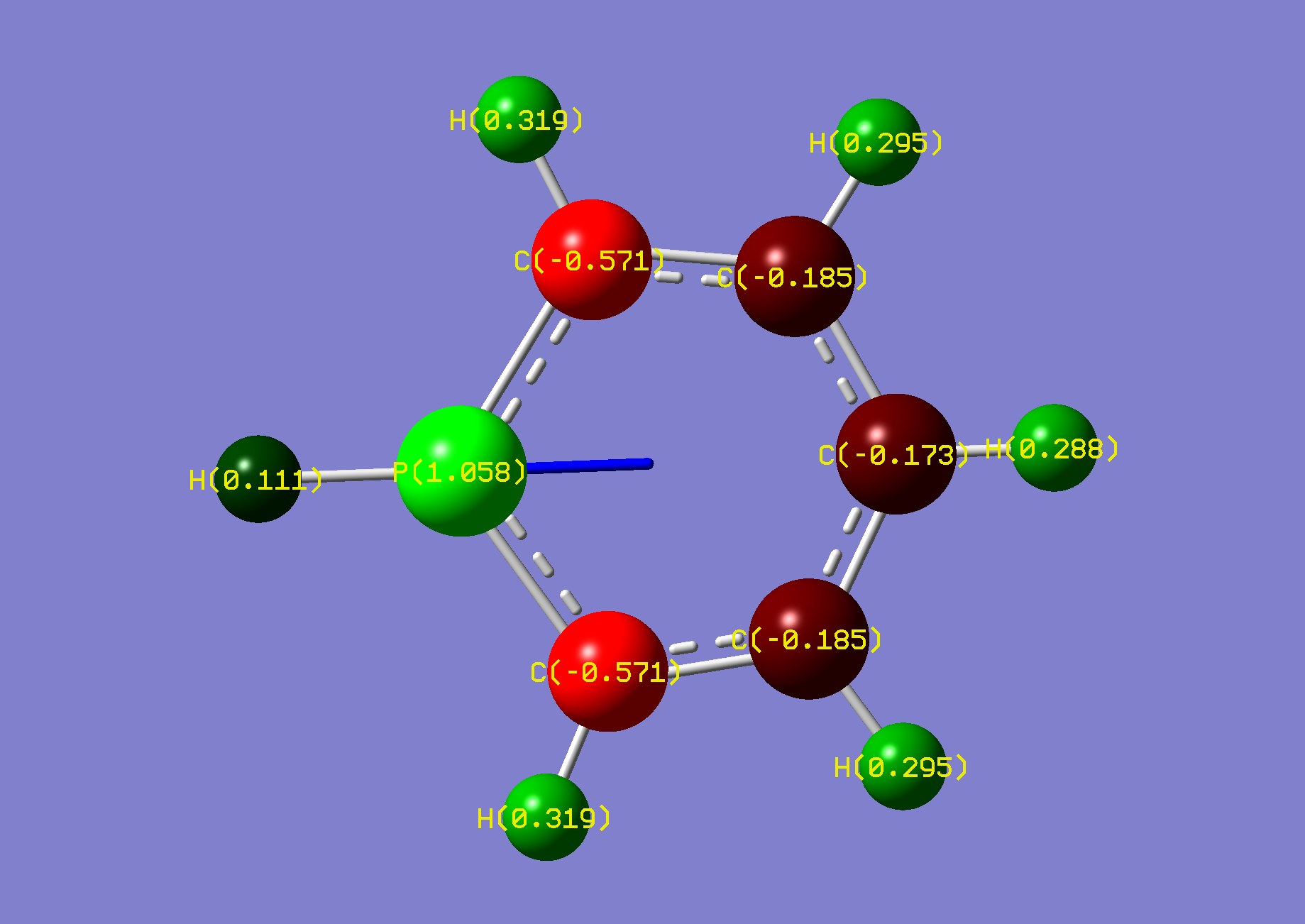
| Name: | 1,2-Diphosphorine: | 1,2-Diphosphorinium: | ||
| NBO Analysis: | 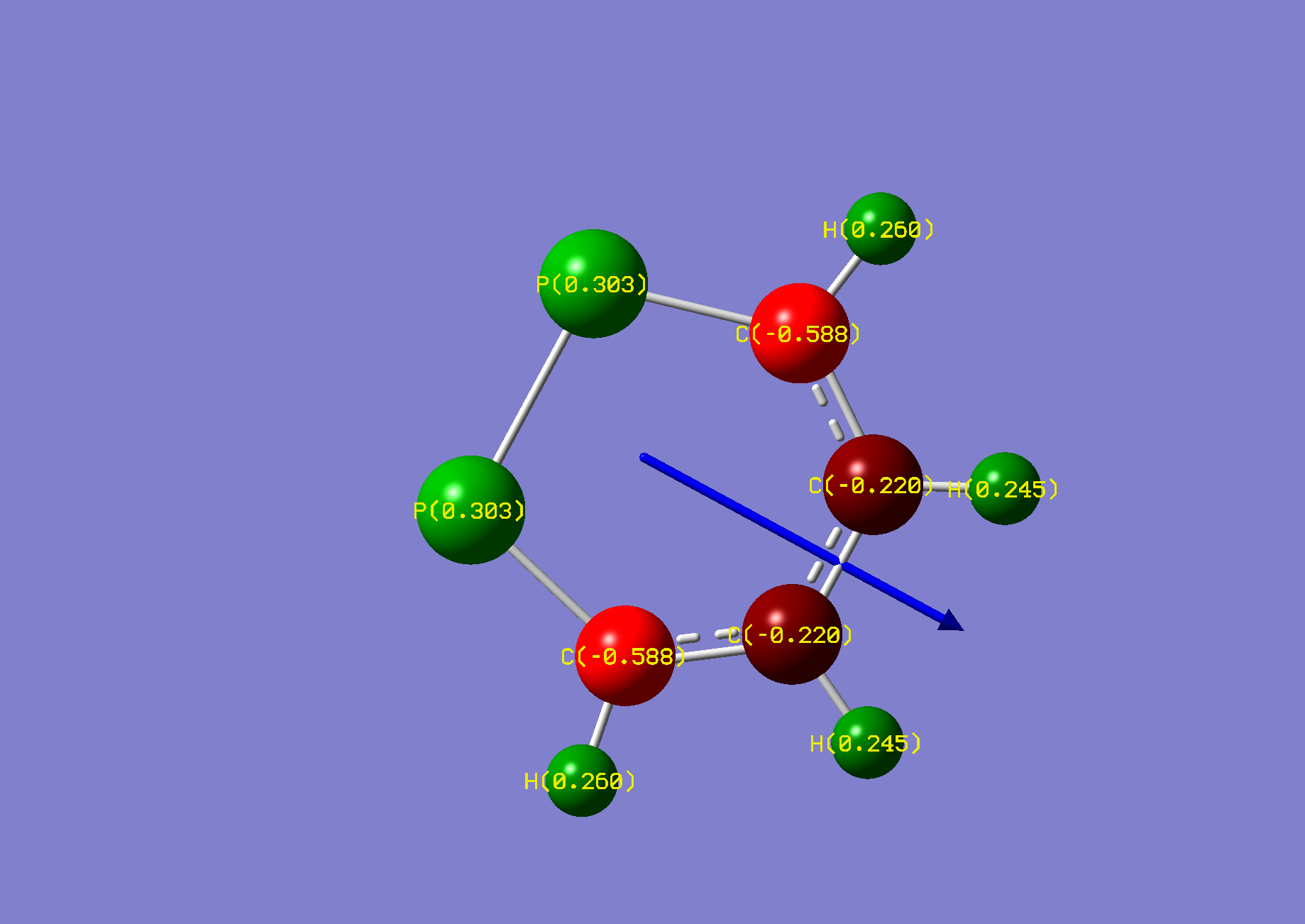 |
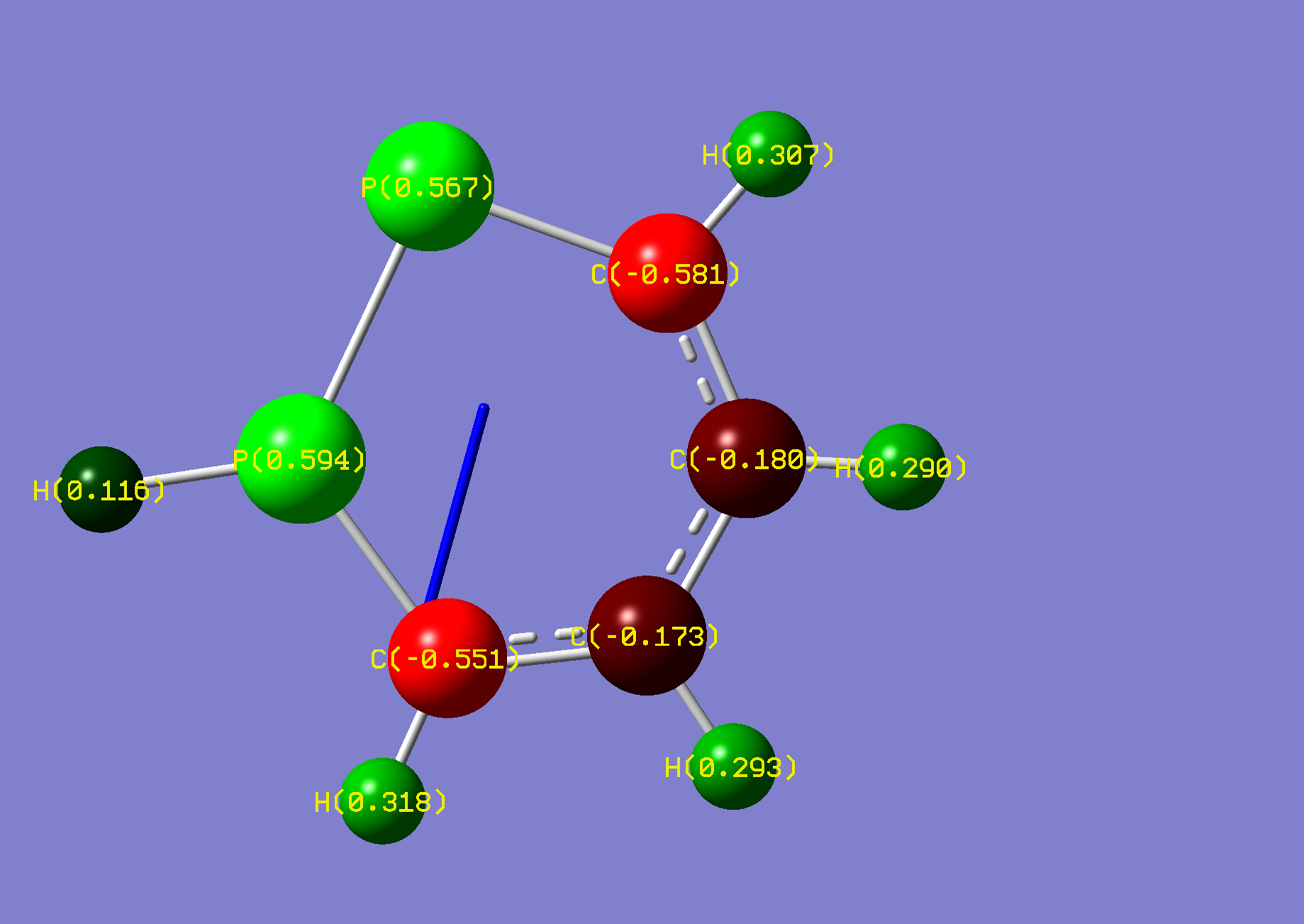 | ||
| Dipole Moment: | 3.0030 Debye | -1.3055 Debye | 1.6975 Debye | |
| Bond angle 6-1-2: | 104.92° | +13.5° | 118.42° | |
| Bond angle 1-2-3: | 129.32° | -9.42° | 119.90° | |
| Bond angle 2-3-4: | 125.76° | +0.01° | 125.77° | |
| Bond angle 3-4-5: | 125.76° | +1.80° | 127.56° | |
| Bond angle 4-5-6: | 129.32° | +3.78° | 133.10° | |
| Bond angle 5-6-1: | 104.92° | -9.68° | 95.24° | |
| Bond length 1-2: | 1.75Å | -0.04Å | 1.71Å | |
| Bond length 2-3: | 1.39Å | 0Å | 1.39Å | |
| Bond length 3-4: | 1.40Å | +0.01Å | 1.41Å | |
| Bond length 4-5: | 1.39Å | 0Å | 1.39Å | |
| Bond length 5-6: | 1.75Å | -0.04Å | 1.71Å | |
| Bond length 6-1: | 2.12Å | -0.03Å | 2.09Å | |
| Energy/kJ·mol-1: | -2,198,926.70 | -857.89 | -2,199,784.60 | |
| Comments: | The dipole moment is reduced and rotated towards the acidic proton, similarly to pyridazine.
The second phosphorus is highly strained. | |||
| Name: | 1,3-Diphosphorine: | 1,3-Diphosphorinium: | ||
| NBO Analysis: | 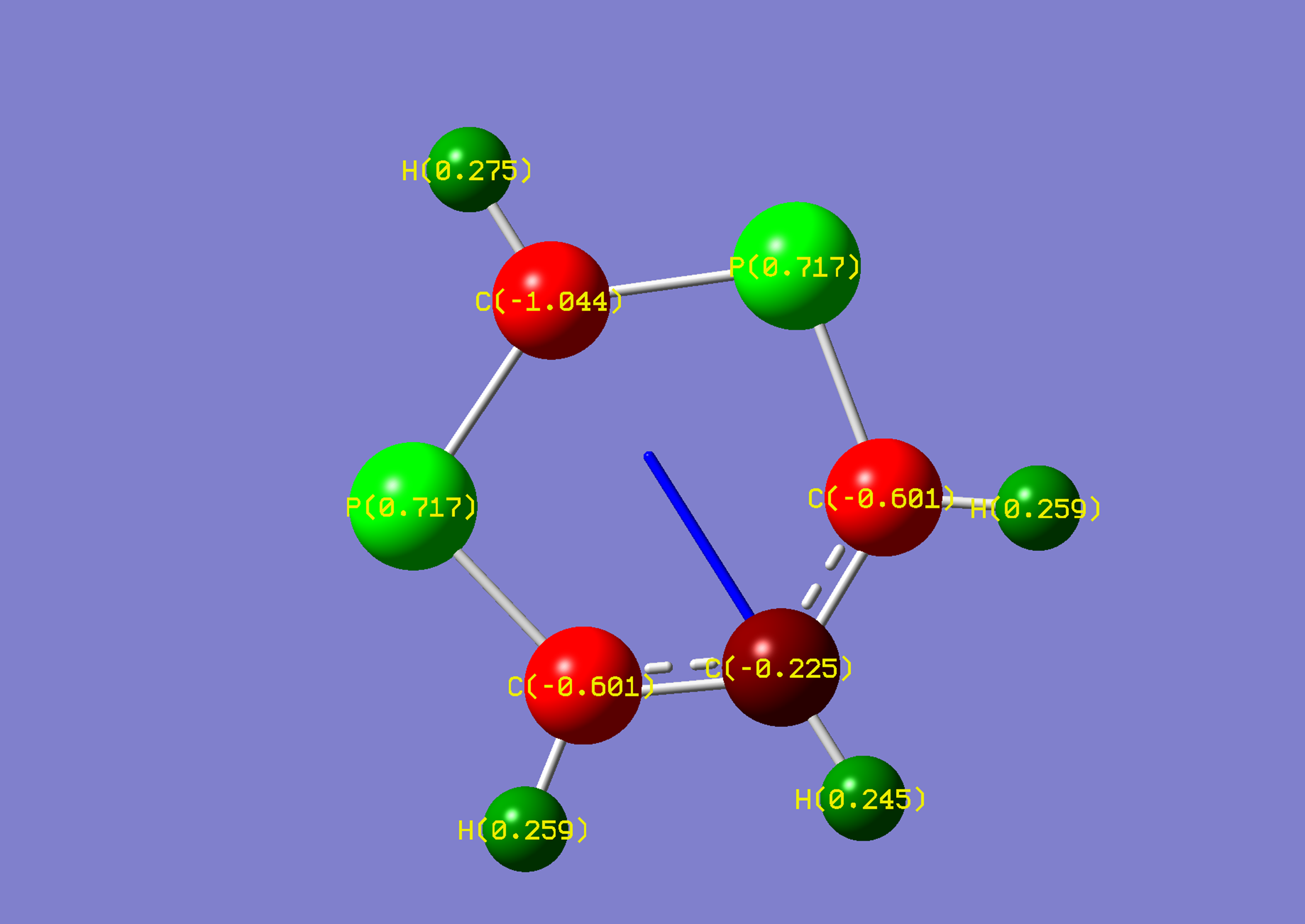 |
 | ||
| Dipole Moment: | 1.5646 Debye | +1.6860 Debye | 3.2506 Debye | |
| Bond angle 6-1-2: | 102.88° | +13.57° | 116.45° | |
| Bond angle 1-2-3: | 131.51° | -8.67° | 122.84° | |
| Bond angle 2-3-4: | 102.88° | +0.22° | 103.10° | |
| Bond angle 3-4-5: | 128.37° | +3.51° | 131.88° | |
| Bond angle 4-5-6: | 125.98° | +0.05° | 126.03° | |
| Bond angle 5-6-1: | 128.37° | -8.66° | 119.71° | |
| Bond length 1-2: | 1.74Å | -0.06Å | 1.68Å | |
| Bond length 2-3: | 1.74Å | +0.02Å | 1.76Å | |
| Bond length 3-4: | 1.75Å | 0Å | 1.75Å | |
| Bond length 4-5: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 5-6: | 1.39Å | +0.01Å | 1.40Å | |
| Bond length 6-1: | 1.75Å | -0.05Å | 1.70Å | |
| Energy/kJ·mol-1: | -2,198,889.93 | -844.33 | -2,199,734.26 | |
| Comments: | The dipole moment is increased and rotated towards the acidic proton, similarly to pyrimidine. Symmetry is lost upon protonation. | |||
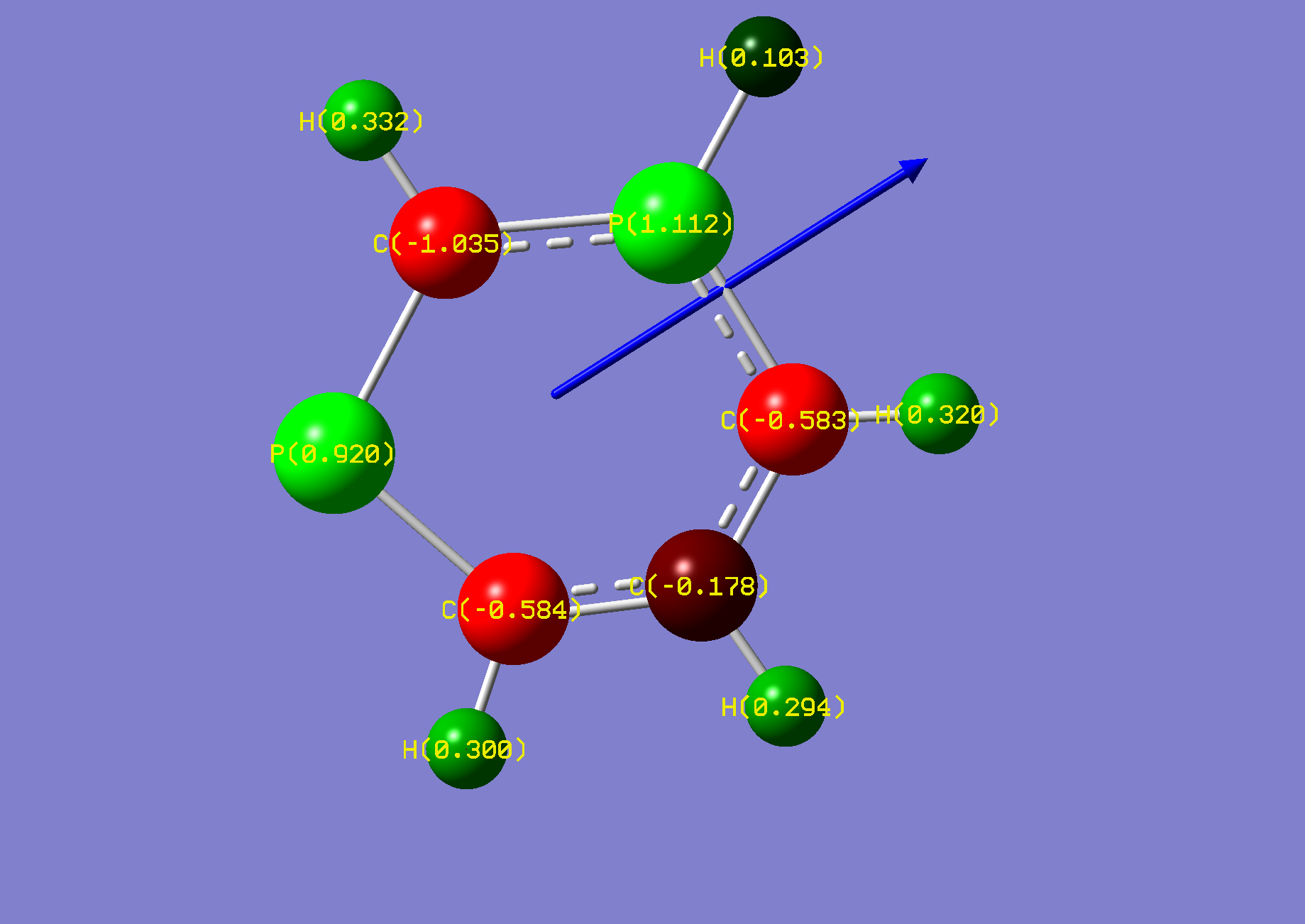
| Name: | 1,4-Diphosphorine: | 1,4-Diphosphorinium: | ||
| NBO Analysis: |  |
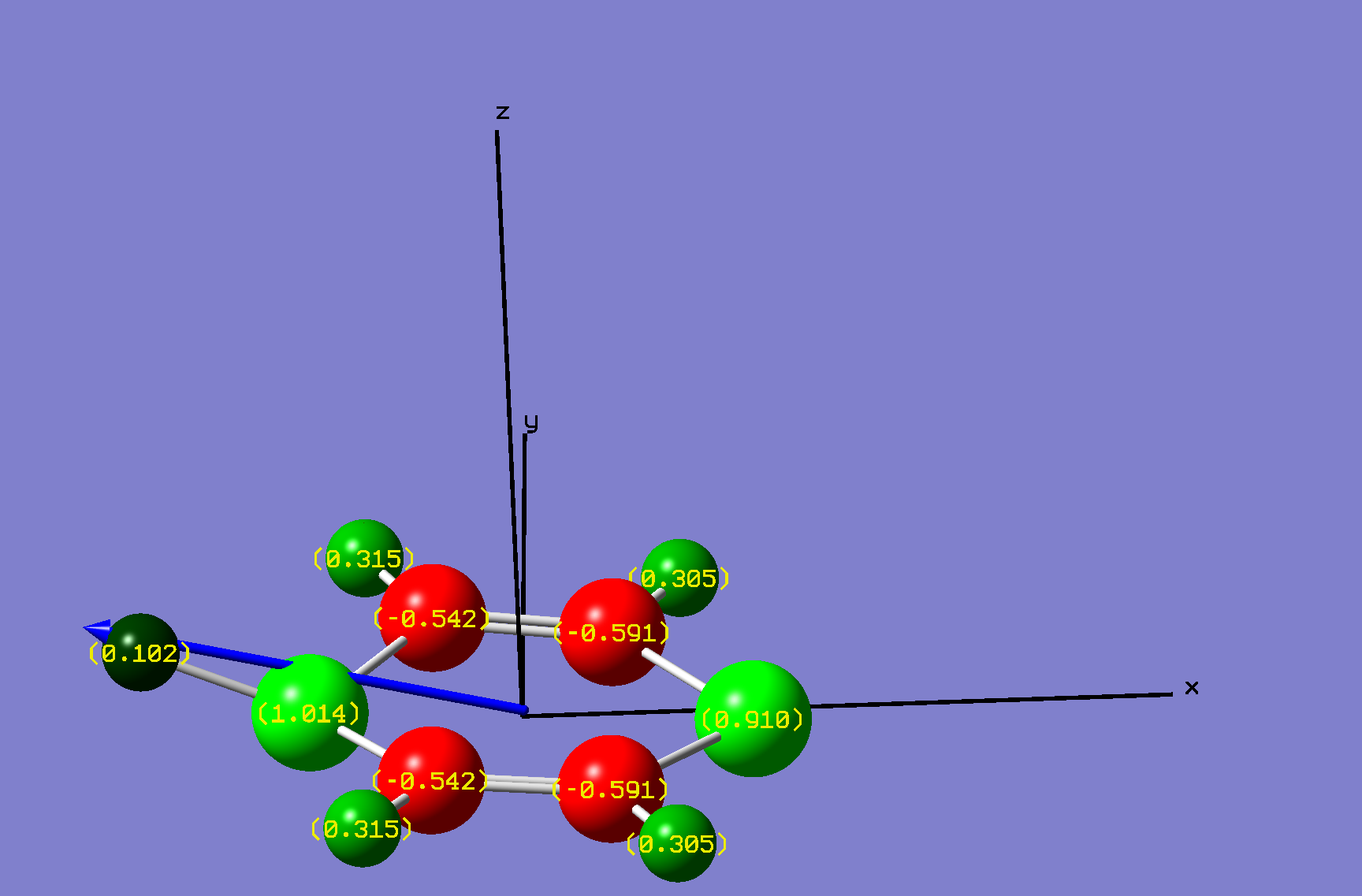 | ||
| Dipole Moment: | 0.0000 Debye | +3.4625 Debye | 3.4625 Debye | |
| Bond angle 6-1-2: | 102.53° | +10.8° | 113.34° | |
| Bond angle 1-2-3: | 128.73° | -8.17° | 120.56° | |
| Bond angle 2-3-4: | 128.73° | +1.49° | 130.22° | |
| Bond angle 3-4-5: | 102.53° | +1.15° | 103.68° | |
| Bond angle 4-5-6: | 128.73° | +1.49° | 130.22° | |
| Bond angle 5-6-1: | 128.73° | -8.17° | 120.56° | |
| Bond length 1-2: | 1.75Å | -0.03Å | 1.72Å | |
| Bond length 2-3: | 1.39Å | 0Å | 1.39Å | |
| Bond length 3-4: | 1.75Å | +0.01Å | 1.76Å | |
| Bond length 4-5: | 1.75Å | +0.01Å | 1.76Å | |
| Bond length 5-6: | 1.39Å | -0.04Å | 1.35Å | |
| Bond length 6-1: | 1.75Å | -0.03Å | 1.72Å | |
| Energy/kJ·mol-1: | -2,198,882.89 | -838.53 | -2,199,721.42 | |
| Comments: | Similarly to pyrazine, a dipole moment is induced. | |||
From the above table, the phosphorus analogues of pyridine and the diazines are similar, except that the protons draw electron density from their neighbouring phosphorus atoms, suggesting a lack of conjugation throughout the systems. As such, the protons are far less acidic.
Phosphinine becomes more aromatic, as can be seen from the conjugation. This could be because the phosphorus becomes more sp3 hydribised. The diphosphorines seem from the results highly unstable. 1,4-diphosphorinium was initially calculated as being in a transition state, and this was resolved later.
Comparing Nitrogen's and Phosphorus' Roles in Aromaticity
The most important difference between nitrogen and phosphorus in this case is their size difference. Nitrogen is relatively similar to carbon, and so is well matched for replacing it in benzene. On the other hand, phosphorus is much larger, and just one causes strain on the system. When a second is added, bonds are distorted far beyond sp3.
The second difference is the energy match and overlap. Nitrogen overlaps well with carbon, as is seen by the huge array of organic nitrogen containing compounds. Phosphorus as a heteroatom is inherently unstable, and it is rare to see such compounds.
Spectra
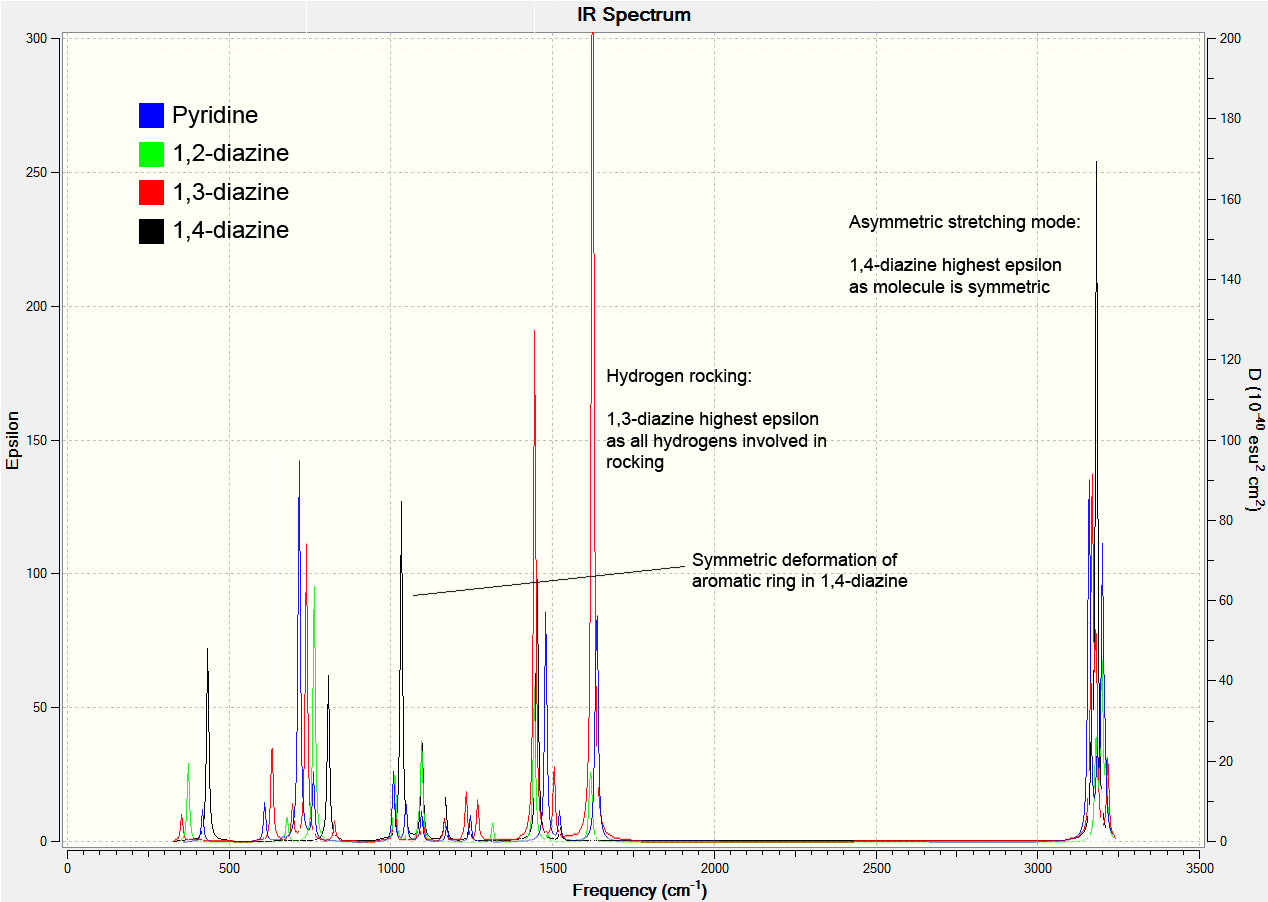
The image below shows the computed spectra for the nitrogen containing molecules. The asymmetric hydrogen stretches are similar for all molecules, except for 1,4-diazine that has a higher maximum due to the symmetry of the compound allowing a synchronisation of the stretches.
The image below shows the computer spectra for the phosphorus containing molecules. The 1,3-diphosphorine splits the hydrogens' asymmetric absorbance bands in two, and so its overall absorbance maximum is lower at this point. However, they are able to rock in unison and show a high absorbance at 1386.09 cm-1.
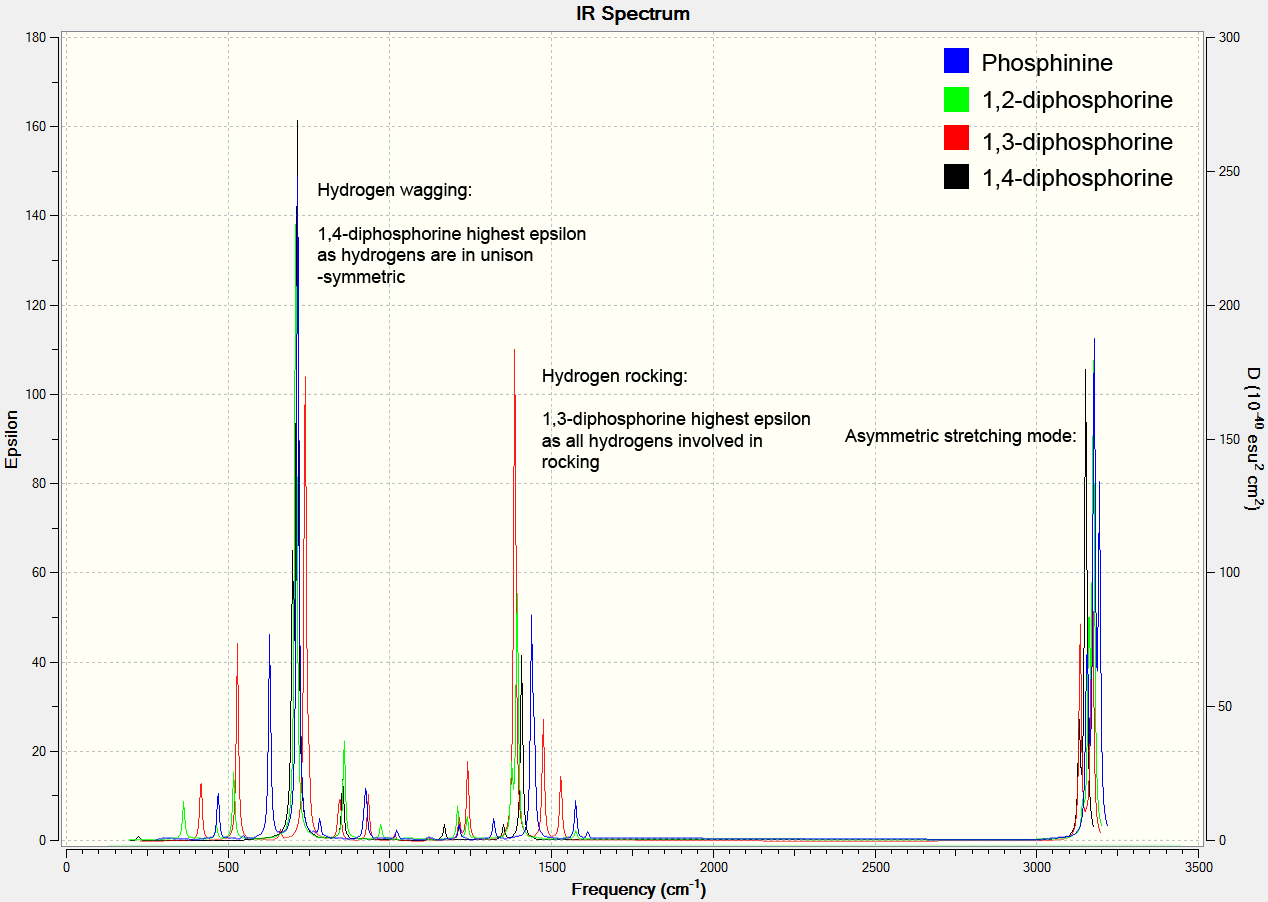
Overall, the hydrogens behave similarly for all the molecules when stretching asymmetrically. Otherwise, they tend to absorb at lower wavenumbers in the phosphorus molecules due to the larger heteroatoms and the weaker bonds. The absorbance was also lower for the phosphorus compounds. Some modes were observed in the nitrogen molecules that weren't in the phosphorus. This could be a breaking of symmetry in the calculations for the nitrogens.
1,4-Diphosphorinium and 1,4-Diazinium
Gaussian calculated the phosphorus hydrogen as being in plane with ring. However, the negative frequency was inspected as vibrating the hydrogen out of plane. Another optimisation was conducted after 'nudging' the hydrogen out of plane manually.
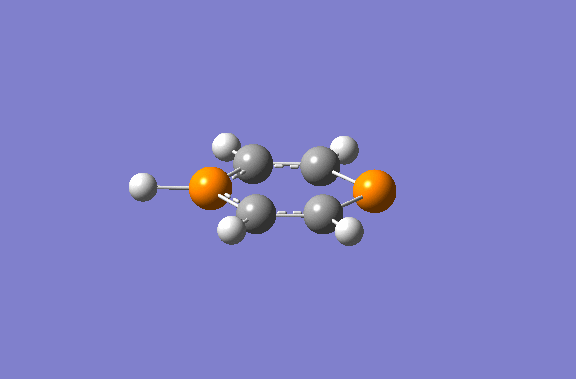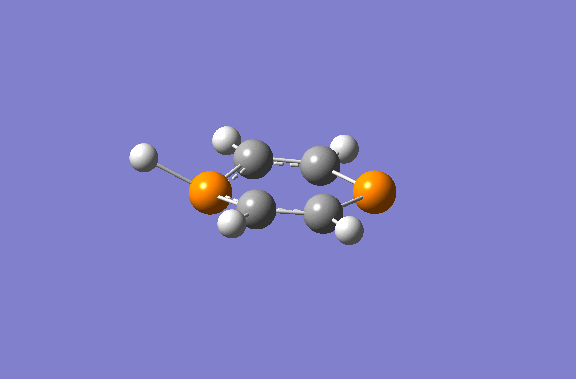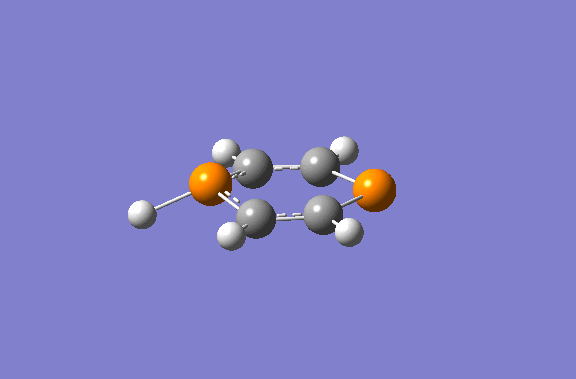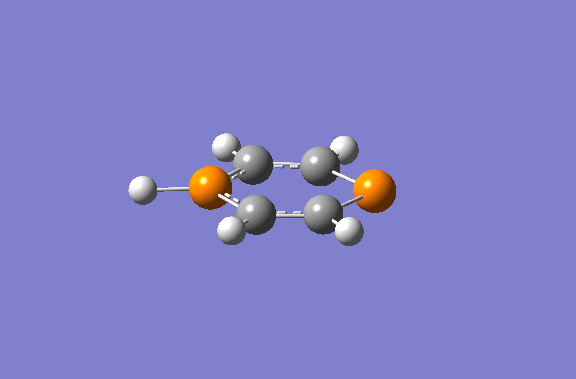
The diphosphorinium was optimised to the following geometry:
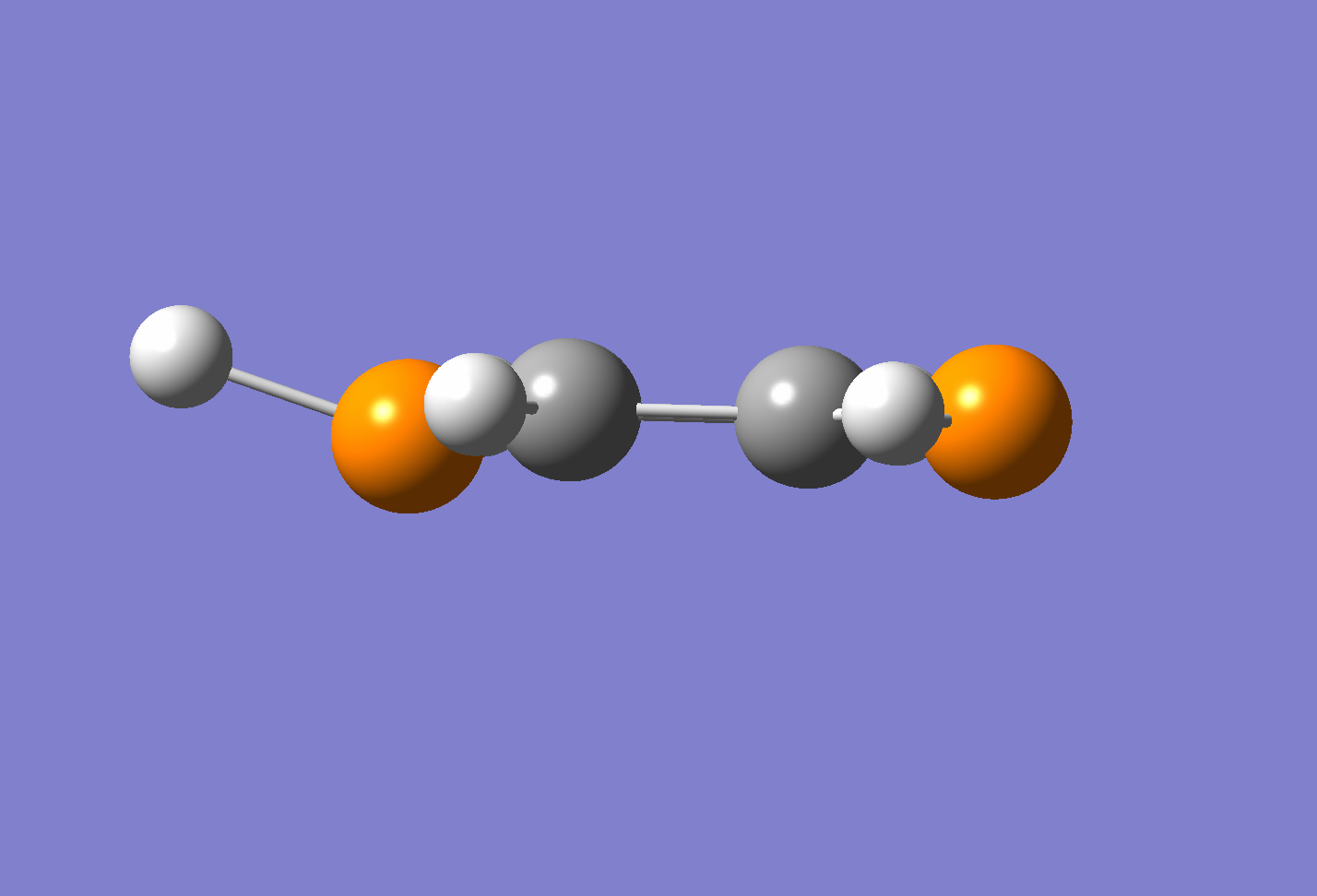
The hydrogen has distorted away from the transition state by over 20°. This shows some mixing between an aromatic system and a diene, with a lone pair causing steric repulsion pushing the hydrogen out of plane. The aromaticity is shown in molecular orbital 25 - a conjugation of the p orbitals along all the ring atoms.
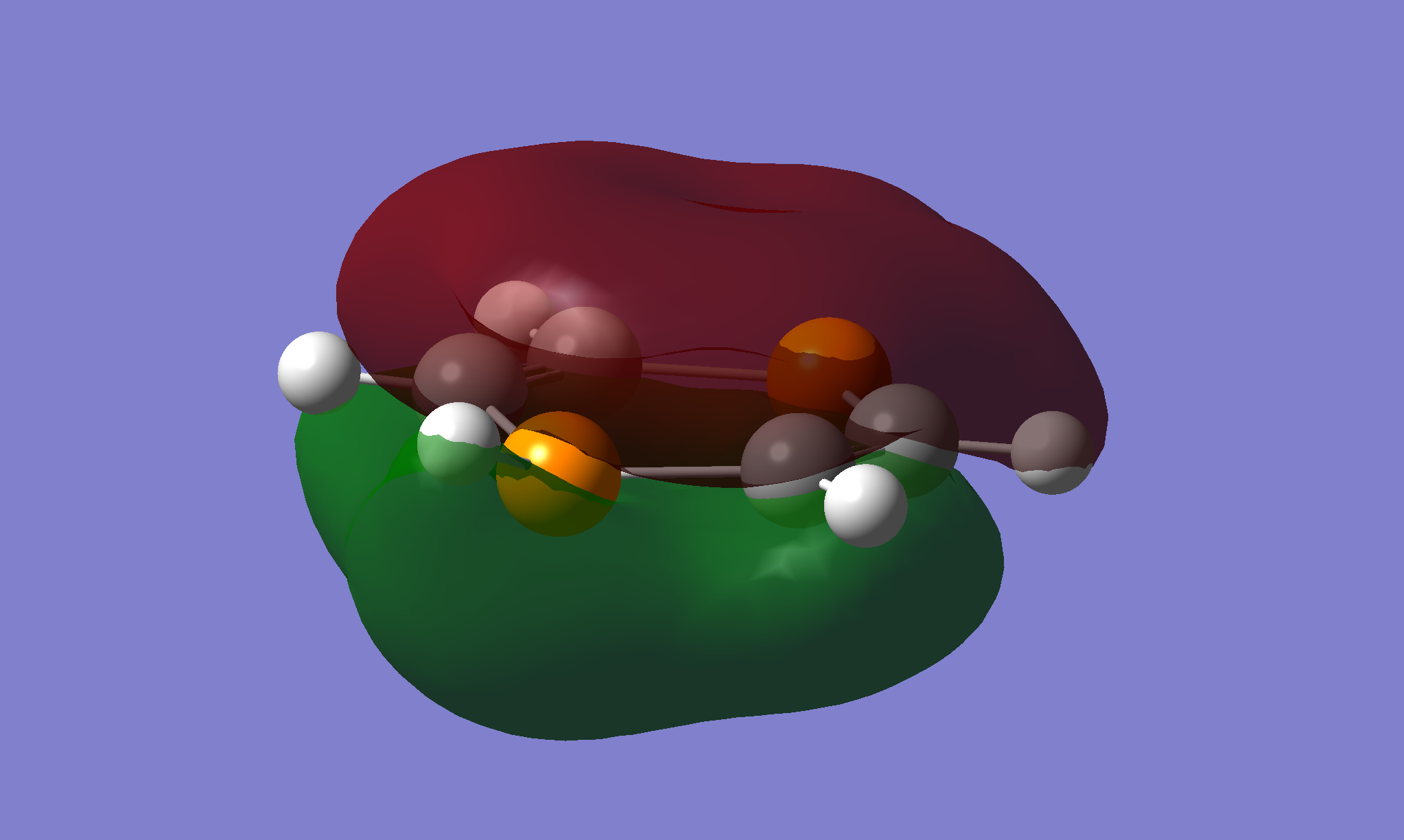
A proposed mechanism for the transition is shown below. The 1,4-diphosphorinium would oscillate between the configurations across a barrier of 0.67kJ·mol-1, calculated as the difference between the local minima and the local maximum transition state.
This transition doesn't exist in 1,4-diazine as it is already the lowest energy configuration. This is because nitrogen is significantly smaller and less polarisable. As well as that, the energy gain from aromaticity is higher than the energy gain of distorting the structure.
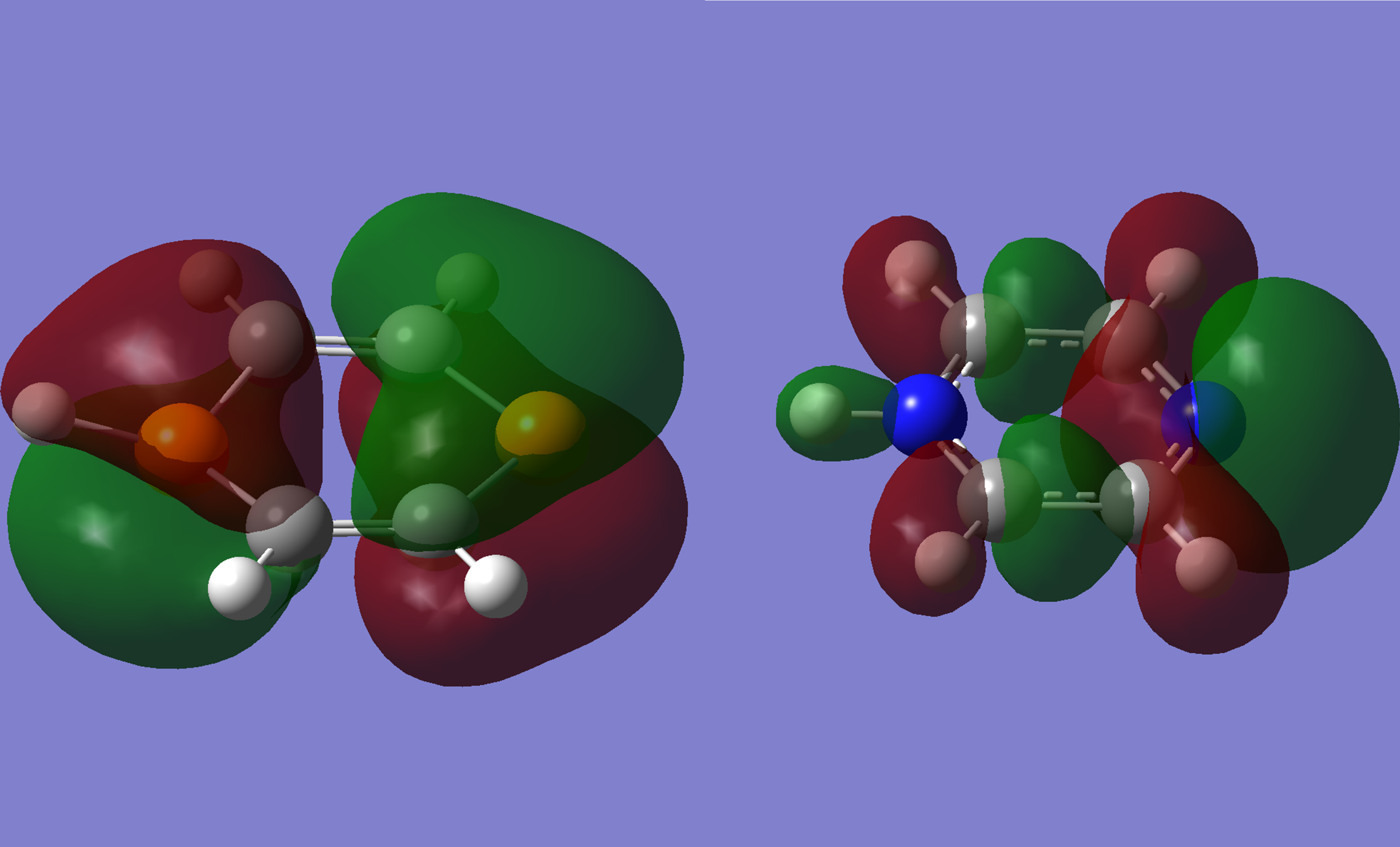
Right - the HOMO of 1,4-diazinium.
The HOMO of 1,4-diphosphorinium is comparable to that of benzene[3], while the 1,4-diazinium is comparable to the highest energy molecular orbital. A lot of the electron density is shown to be withdrawn into the ring for the diazinium.
Conclusions
Despite phosphorus being one below nitrogen in the periodic table, there are notable differences in their heteroaromatic systems:
- A close match in orbital size and energy allows nitrogen to replace carbon fairly well. All the nitrogen containing molecules were considerably more aromatic than the phosphorus analogues.
- Phosphorus strains the bonds in the ring much more than nitrogen, especially when they are beside each other, for geometrical reasons.
- Phosphorus is more electropositive than carbon and nitrogen, and so holds a more positive charge than the nitrogen compounds.
Further Investigation
The following could be computed to expand this project:
- The pKas in the gas phase could be used to compare the molecules.
- Further pnictogens could be investigated to understand how size, orbitals and electronegativity affect the geometric and electronic properties of the compounds.
- Pnictogens could be mixed within the same molecule to see how the charge would be redistributed. The difference in energy for which pnictogen the proton is on could be compared for each compound.
- A second proton could be added to the dipnictogens to demonstrate the influence one heteroatom has on the other.
References
- ↑ E. G. Cox "Crystal Structure of Benzene", Review of Modern Physics, Volume 30, 1958, 159-162DOI:10.1103/RevModPhys.30.159
- ↑ 2.0 2.1 2.2 2.3 Julio Alvarez-Builla, Juan Jose Vaquero, José Barluenga "Modern Heterocyclic Chemistry", Volume 1, 1686DOI:10.1002/9783527637737 10.1002/9783527637737
- ↑ Henry Rzepa "Introduction to Molecular Orbital Theory"