Rep:Mod:00478897
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Objectives of this module of the course:
This experiment involves the use of various computational calculations and molecular modelling to predict the path of chemical reactions and stereochemistry of the products. This module in particular uses molecular mechanics (MM2) to minimise the energy and predict the geometry and regioselectivity of various reactions such as:
- the hydrogenation of cyclopentadiene dimer
- the stereochemistry of nucleophilic addition to two different pyridinium (NAD+ analogues)
- the conformation/atropisomerism of a large ring ketone intermediate in one synthesis of the anti-cancer drug Taxol
- the conformational preferences leading to dramatic (enzyme-like) acceleration of peptide hydrolysis
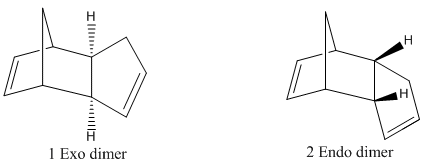
The hydrogenation of cyclopentadienyl dimer
Dimerisation of cyclopentadienyl ring
The reaction involving dimerisation of cyclopentadiene can give two possible products, the exo or endo product. The endo product is believed to be produced specifically but the exo product is thermodynamically more stable as predicted by molecular mechanics after optimising the structure of each product. The results are tabulated as follows:
| Dimer | stretch kcalmol-1 | bend kcalmol-1 | stretch-bend kcalmol-1 | torsion kcalmol-1 | Non-1,4 VDW kcalmol-1 | Non-1,4 VDW kcalmol-1 | Dipole-dipole kcalmol-1 | TOTAL kcalmol-1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.2923 | 20.5870 | -0.8413 | 7.6715 | -1.4358 | 4.2320 | 0.3778 | 31.8834 |
| 2 | 1.2454 | 20.8603 | -0.8320 | 9.5039 | -1.5083 | 4.3012 | 0.4448 | 34.0153 |
The dihedral angles between the two rings for each isomer are shown as follows:
| Dimer | structure | dihedral angle |
|---|---|---|
| 1 | 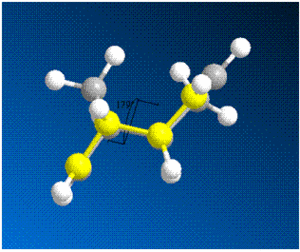 |
179 |
| 2 |  |
-149 |
From the calculations above, it can be deduced that the exo dimer is thermodynamically more stable than the endo dimer. The stability can be explained interms of the dihedral angles as calculated above. The exo-isomer has a dihedral angle of about 180°C which means that the configuration is antiperiplanar and the large carbons groups are at a maximum distance apart. This is not the case with the endo dimer, therefore the exo dimer is thermodynamically more stable. The results from MM2 calculations do not match the experimental data since the endo-product is produced specifically as a result of the dimerisation process. Hence, it can be deduced that the reaction is under kinetic control and not thermodynamic since the stability of the final product does not determine the final result.
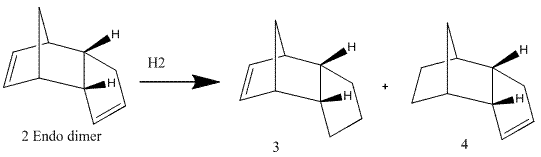
The hydrogenation of cyclopentadiene dimer
The hydrogenation reaction of the endo isomer above can give two possible products:
The energy calculation were performed for both possible products and the results are as follows:
From the calculations, product 4 is more stable as it has a lower energy value. This is probably due to the carbon double bond in 3 being highly constrained due to the presence of the bridging carbons. Product 4 has a sp3 centre near the bridging carbon which allows some movement as the carbons now are not planar and therefore, less strained. This is backed by the relatively higher value of bending energy obtained for product 3 from the MM2 calculations.
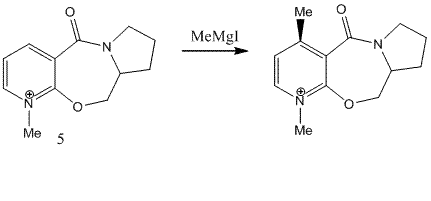
Stereochemistry of Nucleophilic addition to a pyridinium ring
Optically active derivative of prolinol
The energy for 5 was optimised using MM2 without including MeMgI in the calculation since the program gave an error when the Grignard reagent was added to the structure. The results are shown below:
| prolinol derivative 5 | energy component | |
|---|---|---|
| 1.1716 | Stretch | |
| 11.3203 | Bend | |
| 0.0545 | Stretch-Bend | |
| 5.2481 | Torsion | |
| -2.0711 | Non-1,4 VDW | |
| 11.8460 | 1,4 VDW | |
| 2.6980 | Charge/Dipole | |
| -3.9518 | Dipole/Dipole | |
| 26.3156 | Total Energy |
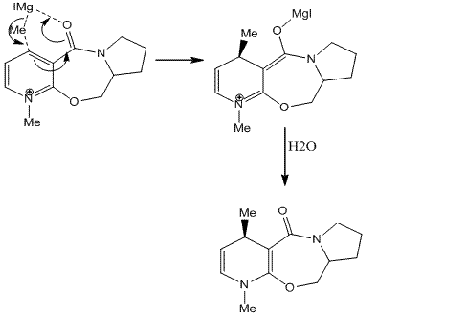
Although the energy has been calculated for the product above, it does not give any information on the stereochemistry observed. The stereochemistry can be explained by having a closer look at the mechanism of the reaction. The Grignard reagent approaches the ring and delivers the methyl group to the carbon of the ring from the top face and chelates to the oxygen atom of the carbonyl group at the same time. As the carbonyl group is pointing out of the plane of paper, the methyl gets added in the same plane. This is shown in the mechanism below [1]:
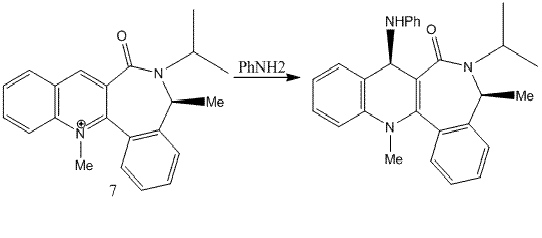
Derivation of pyridinium ring with anilin
The reaction above involves a different nucleophile which is more bulky compared to methyl considered in the previous reaction. The incoming nucleophile does not have the ability to chelate with oxygen atom of the carbonyl group which in this case is pointing the plane of the ring, the nucleophile binds to the pyridinium ring from the opposite face of the carbonyl oxygen. The energy of prolinol derivative 7 was minimised using MM2 and the results are tabulated as follows:
| prolinol derivative 7 | energy component | |
|---|---|---|
| 1.5467 | Stretch | |
| 6.6928 | Bend | |
| 0.3334 | Stretch-Bend | |
| -5.8643 | Torsion | |
| -2.6107 | Non-1,4 VDW | |
| 17.6716 | 1,4 VDW | |
| 2.3202 | Charge/Dipole | |
| -4.8248 | Dipole/Dipole | |
| 15.2649 | Total Energy |
Stereochemistry and reactivity of an intermediate in the synthesis of taxol
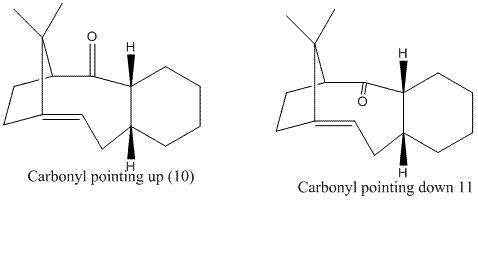
Atroptisomers and their stability
This section involves the study of two intermediate compounds linked by atropisomerism in the total synthesis of Taxol.
The stereochemistry of the carbonyl with respect to the bridging carbon determines which isomer is more stable. Both isomers were optimised using MM2 and the results are tabulated below:
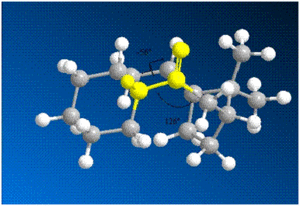
From the calculations, the atropisomer with the carbonyl pointing down with respect to the bridging carbon (11) is more stable. The reason for this stabilisation could be the 1,3 diaxial interaction present in 10 between the atoms marked yellow which is absent or reduced in 11. The instability of isomer 10 can also be attributed to the bond angle illustrated in the diagram above. The carbonyl bond is stretched to 126° which is not characteristic of the carbonyl bond 120°. Whereas the carbonyl bond angle for isomer 11 is (120°).
Hyperstability of Alkenes
To prove that isomer 10 undergoes reactions slowly, the energy of the hydrogenated product was minimised using the MM2 program. The energy for the product was proven to be significantly higher compared to 10.
| carbonyl up (hydrogenated) | energy component | |
|---|---|---|
| 3.2593 | Stretch | |
| 19.7348 | Bend | |
| 0.6433 | Stretch-Bend | |
| 21.9517 | Torsion | |
| 1.6318 | Non-1,4 VDW | |
| 16.6862 | 1,4 VDW | |
| 0.0000 | Dipole/Dipole | |
| 63.9070 | Total Energy |
The concept of hyperstable alkenes was first brought by Schleyer in 1981. According to the definition, hyperstable olefins are less strained than the corresponding hydrogenated products and therefore, show an increase in energy when a reacted with hydrogen [2]. In this case, the process of hydrogenation is disfavoured due to the formation of unfavourable 10 membered ring and the presence of rigid and bulky bridging carbon atom which causes steric hindrance. Due to this, only one end of the alkene may be approached by the electrophile whereas the other end adjacent to the bridging group is rendered inaccessible.
How one might induce room temperature hydrolysis of peptide
This exercise demonstrates the use of molecular mechanics to investigate the kinetic behaviour of molecules based on the different conformations adopted by them. The reactions under consideration is the intramolecular reaction, hydrolysis of a peptide bond and the effect of the relative orientation of the OH bond on the rate of the reaction.
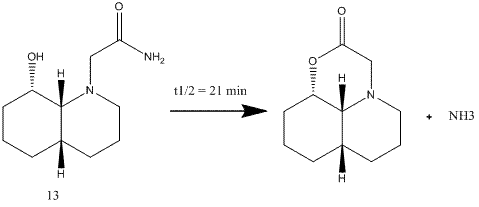
For the compound labelled 13, there are two possible isomers when the hydroxide group is in the equatorial position. The peptide substituent may take the axial or equatorial position, therefore giving two possible isomers with different energies. The energy for both was minimised using the MM2 program and the results are as follows:
| Isomer | Position of OH | Position of peptide | stretch kcalmol-1 | bend kcalmol-1 | stretch-bend kcalmol-1 | torsion kcalmol-1 | Non-1,4 VDW kcalmol-1 | Non-1,4 VDW kcalmol-1 | Dipole-dipole kcalmol-1 | TOTAL kcalmol-1 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| equatorial | equatorial | 1.7082 | 5.1115 | 0.5331 | 9.5372 | -7.4312 | 10.0677 | -6.3644 | 13.1621 | |||
| equatorial | axial | 1.6673 | 8.2997 | 0.6441 | 11.9053 | -7.9726 | 10.1859 | -4.9198 | 19.8098 |
As expected, the energy for the isomer with amide in the equatorial position is lower than the axial one due to the 1,3 diaxial compression present in the axial isomer. The structures were manipulated so as to give the minimum energy. The hydroxide group was placed in such a way which allowed the hydrogen atom of the OH group to bond with the oxygen atom of the carbonyl group via H-bonding.
The second reaction under consideration is as follows:
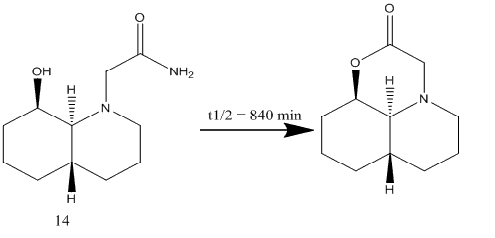
The compound 14 can also exist in possible isomeric forms containing the peptide group in the axial or equatorial positions. The energies were minimised using molecular mechanics:
| Isomer | Position of OH | Position of peptide | stretch kcalmol-1 | bend kcalmol-1 | stretch-bend kcalmol-1 | torsion kcalmol-1 | Non-1,4 VDW kcalmol-1 | Non-1,4 VDW kcalmol-1 | Dipole-dipole kcalmol-1 | TOTAL kcalmol-1 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| axial | equatorial | 1.4696 | 3.8935 | 0.5045 | 7.7128 | -7.1706 | 9.9107 | -6.6078 | 9.7127 | |||
| axial | axial | 1.6781 | 5.4117 | 0.6034 | 8.8213 | -5.2117 | 9.5668 | -6.8429 | 14.0267 |
The equatorial isomer is again more stable with lower energy due to the 1,3 diaxial compression in the axial isomer.
Comparing the energies of these isomers with those calculated above, it can be deduced that 14 is overall more stable than 13. This is probably because the OH group takes the axial position in 13 and therefore, increases 1,3 diaxial compression.
The hydrolysis reaction involves the nucleophilic attack by the lone pair on the oxygen atom of OH group. In order for this reaction to take place, the OH group and the peptide group should lie in the same plane [3]. In 13, both these groups are equatorial and therefore, lie in the same plane. This is not the case for 14 where the hydroxide group is axial to the equatorial peptide group. For the hydrolysis reaction to happen, the peptide may have to change from equatorial to axial position which results in a high energy transition state and is disfavoured. Therefore 14 undergoes reaction very slowly.
The hydrolysis reactions considered in this section take place faster than the normal esterification of a peptide because these reactions are taking place intramolecularly. Normal reactions are intermolecular and are much slower since they are dependent on other factors such as the collision of two or more molecules and their energies as well as the overlap.
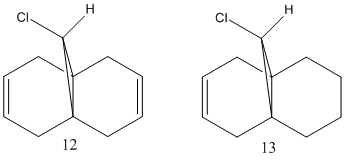
Regeoselective addition of dichlorocarbene
This excercise illustrates the study of reaction of the molecule shown below with electrophilic reagents like carbenes and peracids. This sections also illustrates transition from a purely classical mechanical treatment to quantum mechanical treatment.
The first part of the exercise involved optimisation of the geometry using MM2 calculations followed by Hartree-Fock (HF) program along with STO-3G basis set. The HF method enabelled us to calculate the molecular surfaces of the molecule such as HOMO and LUMO which can be used to predict the reaction of this compound with electrophiles.
The second part requires the comparison of 12 with the hydrogenated version (13) of this molecule which contains one of the C=C bonds replaced by a C-C bond. The geometries of both molecules were optimised using MM2 and HF calculations and a much accurate program density functional theory (DFT) method. The results were then used to calculate the vibrational frequencies.
| Compound (12) | C-Cl bond angle (MM2) | C-Cl bond angle (HF) | C-Cl bond angle (DFT) |
|---|---|---|---|
 |
118 | 122 | 122.7 |
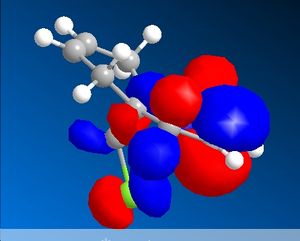
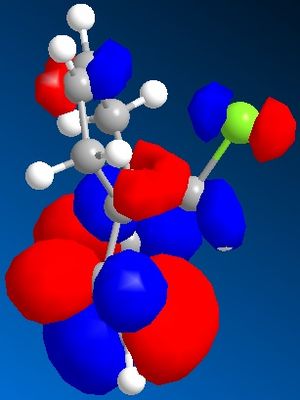
The C-Cl-C(ring) bond angle calculated using MM2 is quite different to the one calculated from HF and DFT which shows that MM2 is less accurate compared to the other techniques. The molecular surfaces obtained from HF technique are as follows:
| HOMO | HOMO -1 | LUMO | LUMO+1 | LUMO +2 |
|---|---|---|---|---|
 |
 |
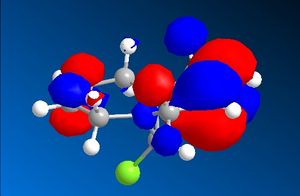 |
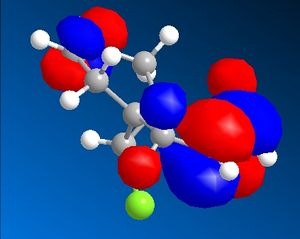 |
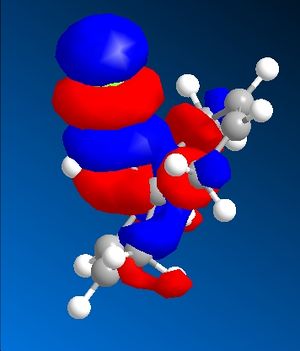 |
The attack by electrophiles can be predicted to take place at the most electron rich alkene. From the molecular surfaces above, the electron density lies around the endo alkene in the HOMO orbital. This argument is further backed by the stabilisation due to antiperiplanar interactions between the C-Cl σ* orbital (LUMO+2) and occupied exo C=C π orbital (HOMO-1)orbital. This renders the endo C=C even more nucleophilic. This interaction results in the exo double bond being pushed towards the bridgehead carbon as proven by the distances obtained from our calculations. The distance from bridgehead obtained for exo is 3.018 whereas for endo is 3.235 angstroms [4].
The DFT calculations for both compounds, 12 and 13 were performed and the resulting optimised structure was then used to obtain the stretching frequencies of functional groups present in these molecules. The table below shows the strecting frequencies of C-Cl and C=C bonds for both molecules.
| Compound | C-Cl stretch | C=C (exo stretch) | C=C (endo) stretch | ||
|---|---|---|---|---|---|
| 772.634 | 1740.76 | 1760.91 | |||
| 782.383 | - | 1757.6 |
The C=C bond stretching frequency for the exo C=C is lower due to the interactions with the C-Cl antibonding orbitals which causes a loss of electron density. As the electrons are fed into the anti-bonding orbital, the C-Cl bond weakens and therefore the stretching frequency goes down. Hence, when this C=C double bond is hydrogenated, the C-Cl frequency increases.
Mini Project
Mechanisms of nucleophilic substitution of cyclic acetals:
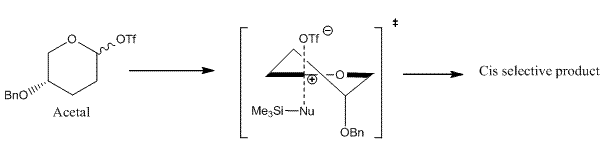
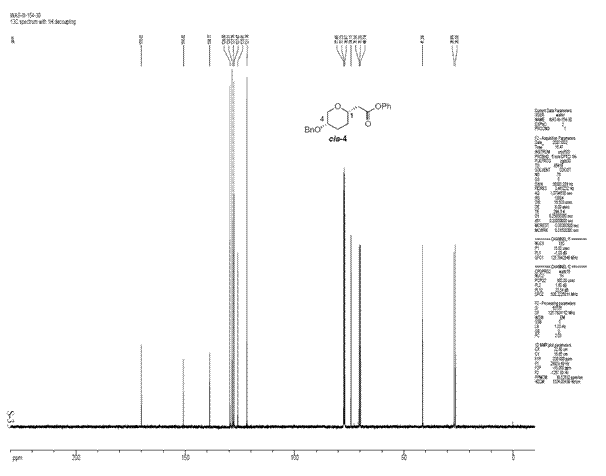
Majority of the organic reactions carried out in the laboratory give mixtures of products which may be isomers of each other. In this section, a reaction will be considered which gives potentially two or more possible isomers which can be distinguished from each other through spectroscopic techniques such as 13C NMR, infra red and Mass spectroscopy after being isolated. For this exercise, the following reaction was chosen in which an acetal upon reaction with a nucleophile gives two possible stereoisomers[5].
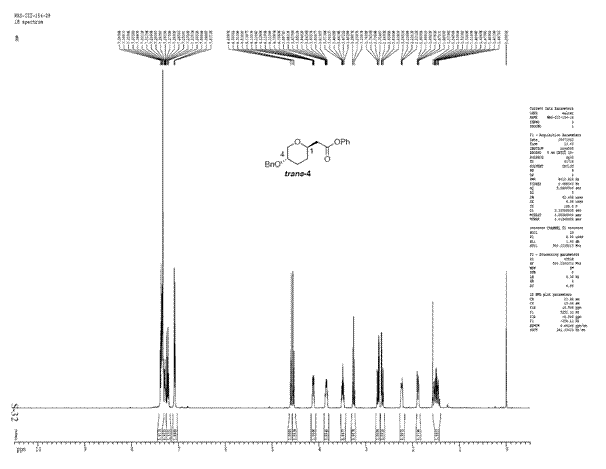
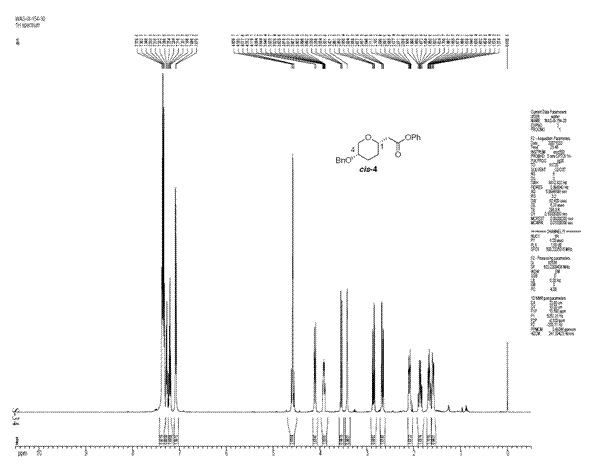
The ratio of cis to trans product obtained in this reaction was 71:29. The cis selectivity observed can be explained if the reaction is considered to proceed via an SN2 like substitution with the transition state shown below.
As the triflate group departs, a partial positive charge builds up on the carbon being attacked by the nucleophile. In order to stabilise the positive charge, benzyloxy group adopts an axial orientation [5]. The benzyloxy group donates electron density due to inductive effect which involves the lone pair of the oxygen atom. The partial positive charge on the carbon atom can be thought of as an empty p orbital perpendicular to the heteroaromatic ring. For an effective overlap between the lone pair of oxygen atom and this empty p-orbital, the benzyloxy group attains an axial orientation. The overlap would not have been as effective if the benzyloxy group was equatorial instead of axial.This accounts for the selectivity observed in this reaction. To see if we can account for this selectivity observed using the molecualr mechanics program, the structures of both isomers were minimised using MM2 and the results are tabulated below:
From the results above, cis isomer which is formed in excess has a lower energy but the difference is negligible although the results do favour the formation of cis isomer selectively. Looking at the reaction conditions used in the literature, it can be said that the reaction is kinetically controlled as the temperatures used are extremely low. The isomer formed as a result (cis) is the knetically more stable product.
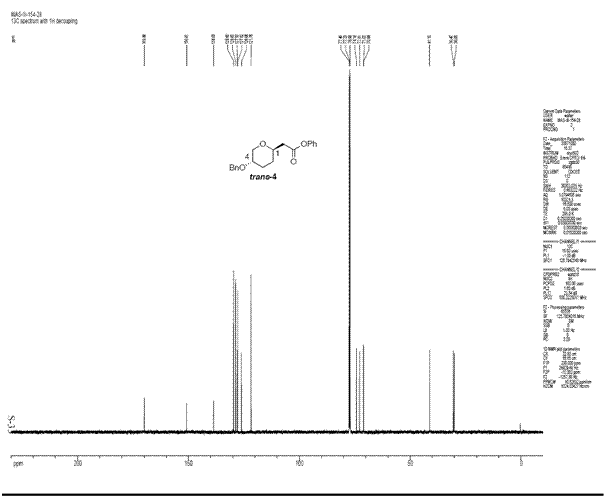
The structures of both isomers above were optimised by MM2, followed by HF/STO-3G calculations and finally by DFT=mpw1pw91/6-31G. It was then sent for scan to obtain the final 13C NMR spectra which was compared to those found in the literature. The spectroscopic analysis and the optimised structures are as follows:
| Cis isomer | trans isomer | ||||
|---|---|---|---|---|---|
| Cis isomer | Cis isomer (literature) | Trans isomer | Trans isomer literature |
|---|---|---|---|
| 163.928 (8) | 170.0 | 163.659 (8) | 169.9 |
| 146.414 (19) | 150.8 | 146.324 (19) | 150.8 |
| 136.405 (11) | 138.8 | 136.502 (11) | 138.6 |
| 125.98 (21) | 129.5 | 125.856 (21) | 129.6 |
| 125.354 (23) | 128.5 | 125.367 (23) | 128.7 |
| 124.520 (15) | 127.8 | 124.866 (13) | 127.9 |
| 124.104 (13) | 127.7 | 124.188 (15) | 127.8 |
| 122.227 (14) | 125.9 | 123.978 (16) | 126.1 |
| 121.601 (22) | 121.8 | 123.865 (14) | - |
| 120.559 (12) | - | 123.074 (12) | - |
| 120.559 (16) | - | 121.588 (22) | 121.8 |
| 118.265 (24) | - | 118.391 (24) | - |
| 115.972 (20) | - | 116.130 (20) | - |
| 73.436 (4) | 74.1 | 73.016 (4) | 74.2 |
| 71.143 (1) | 70.4 | 69.674 (17) | 72.8 |
| 70.726 (2) | 70.2 | 68.483 (2) | 71.0 |
| 67.807 (17) | 69.7 | 68.307 (1) | 70.9 |
| 44.037 (7) | 41.4 | 43.358 (7) | 41.4 |
| 26.731 (5) | 27.0 | 31.454 (6) | 30.5 |
| 22.264 (6) | 26.4 | 30.201 (5) | 30.1 |
The 13C NMR data obtained from this exercise is pretty close to the literature given that this technique is not very accurate especially for carbon atoms bonded to heteroatoms, carbonyl of esters and amides. Most of the chemical shifts obtained are within 5ppm values of the literature data. Another thing to notice is that some chemical shifts which are very close to each other are missing from the literature data. This may be due to overlapping signals observed in the NMR spectra by the researchers which could not be rationalised properly as independent signals. The literature NMR spectra and IR data for both isomers are also attached as follows [5]:
| IR frequency(Cis Lit.) | IR frequency (Trans Lit) |
|---|---|
| 3066 | 3064 |
| 3031 | 3033 |
| 2944 | 2939 |
| 2858 | 2863 |
| 1756 | 1756 |
| 1594 | 1594 |
How to differentiate between two isomers
Unfortunately, for the example discussed above, spectroscopy techniques such as 13C and 1H NMR cannot help separate isomers from each other if samples of each were provided with unknown identity. The coupling constants from the proton NMR obtained from the literature show similar values for the protons connected to the benzoloxy and the ester group. This makes it impossible to differentiate the cis and trans isomers. Similarly, the literature values of IR stretches for both isomers show no significant difference and hence, can not be used to identify unknown samples of each isomers. Therefore, the geometry optimisation to obtain IR frequencies was not performed as part of this exercise.
A very useful technique would be X ray crystallography. This would give the precise nature and position of the atoms but will only work if both isomers can be crystallised separately.
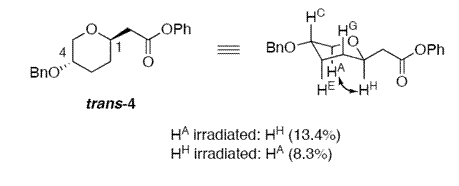
However, another useful technique based on spectroscopy discussed in the literature is the nuclear overhauser effect (NOE). In magnetic resonance spectroscopy, the transfer of spin polarisation from one spin population to another via cross relaxation is known as Overhauser Effect [6]. NOE is observed through space and not through bonds. The amount by which a signal is enhanced is directly proportinal to 1/R6 where R is the distance between the atoms under observation. Usually, distances greater than 2.6 angstroms do not exhibit the overhouser effect, hence no signal enhancement is observed. This observation can be exploited and used to rationalise the structure of the six membered ring in this exampple and the relative orientation of the hydrogen atoms and substituents attached to this ring. The experimental NOE values obtained by the researches in the literature are as follows:
The diagrams above show the experimentally obtained values of NOE. When HA was irradiated, the signals for HC, HE and HH protons were enhanced which means that HA is in close proximity to all these protons. The hydrogen of interest here is the HC proton which has experienced an enhancement of signal by 13 %. The spacial distance of HC obtained from the optimised structure is 2.42 angstroms from HA. If HC took the axial position instead of the equatorial as shown above, this signal enhancement would not have been observed since the spacial distance between HA and HC would have been greater (~3.2 angstroms calculated from optimised structures). Similarly, the position of HH proton was predicted using the results from irradiation of HA proton.
For the trans isomer, irradiation of the HA proton lead to the signal of HH proton being enhanced only and not HC proton. Thus it can be concluded that HC is axial instead of equatorial which would have a smaller spacial distance from HA proton. According to these results, HH can easily be predicted to be in the axial position instead of equatorial.
From the data above, we can approximately predict the position of the substituents in our molecules and hence differentiate between two unknown samples of isomers.
Conclusion
This module has illustrated the usefulness of various computation techniques which have been used to predict the paths of chemical reactions and determine whether they are under kinetic or thermodynamic control. The relative stability of compounds was also predicted using molecular mechanics programs. Lastly, computational mechanics was proved to be a vital technique in predicting NMR spectra for compounds and hence differentiate between isomeric products.
References
- Arthur G. Schultz, Lawrence Flood, James P. Springer, J. Org. Chem., 1986, 51(6), 838-841.
- Van Eis, Maurice J.; Wijsman, Geerlig W.; De Wolf, Willem H.; Bickelhaupt, Friedrich; Rogers, Donald W.; Kooijman, Huub; Spek, Anthony L. Hydrogenation of [5]- and [6]metacyclophane: reactivity and thermochemistry. Chemistry--A European Journal (2000), 6(9), 1537-1546.
- Nicolette M. Fernandes, Fabienne Fache, Mari Rosen, Phuong-Lan Nguyen, and David E. Hansen J. Org. Chem., 2008, 73 (16), 6413-6416
- B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447.
- Jennifer R. Krumper, Walter A. Salamant, and K. A. Woerpel., Org. Lett., 2008, 10 (21), 4907-4910
- http://en.wikipedia.org/wiki/Nuclear_Overhauser_effect (Accessed on the 3rd of February).