Rep:Mod3clm08TC
The Cope Rearrangement
In this section the possible transition states for the cope rearrangement have been modelled and analysed.
1,5-hexadiene
Initially the Anti-Linkage and Gauche 1 conformations were optimised using the HF method and the 3-21G basis set. Once optimised the energies of the two molecules was examined, as can be seen form the table above, the gauche conformation is slight more stable that the anti-linkage conformer. A second gauche conformation was then defined and optimised the energy of which is significantly lower. The energy and point group matches with the lowest energy conformation found in the appendix of the lab manual. This conformation is the most stable as it maximise the attractive VDW H-H interactions (2.4Å[1]), it also minimises steric clashing.
The anti-linkage conformation below was then optimised using the HF method and the 3-21G basis set. The total energy of the molecule is -231.69254 a.u. which is exaclty the same as that which is recorded in the lab manual, this suggests that optimisation of the correct geometry has been completed. It was then optimised again using the DFT method and a B3LYP/6-31G* basis set. The table below shows a comparison between the geometries of the optimised structures.
| HF/3-21G | B3LYP/6-21G | ||
|---|---|---|---|
| Bond Length/Å | C=C | 1.32 | 1.33 |
| C-C | 1.55 | 1.55 | |
| C=C-H | 1.07 | 1.09 | |
| C-H | 1.08 | 1.10 | |
| Angle/° | C=C-C-C-C=C | 111.4 | 112.6 |
| C=CH2 | 116.3 | 116.5 | |
| C=C-CH2-C-C=C | 107.7 | 106.7 | |
| C=CH-C-C-C=C | 115.5 | 115.7 | |
There is very little difference between the geometries of the two optimised structures. This indicates that the use of a lower level calculation is a good approximation in this case, and therefore computational time is shorter.
A frequency analysis of the higher level optimisation (B3LYP/6-31G*) yields the following values:
Sum of electronic and zero-point Energies= -234.469180 a.u. Sum of electronic and thermal Energies= -234.461842 a.u. Sum of electronic and thermal Enthalpies= -234.460898 a.u. Sum of electronic and thermal Free Energies= -234.500730 a.u.
The energies corrected for 298.15K are:
Thermal correction to Energy= 0.154744 Thermal correction to Enthalpy= 0.155688 Thermal correction to Gibbs Free Energy= 0.121355
Two CH2CHCH2 (C2h) fragments were orientated into a chair transition state and optimised to a transition state initially using HF/3-21G and then by fixing the distance between the terminal carbons on the fragments to 2.2Å, optimising to a minimum HF/3-21G and then introducing a bond between the terminals and optimising to a transition state.
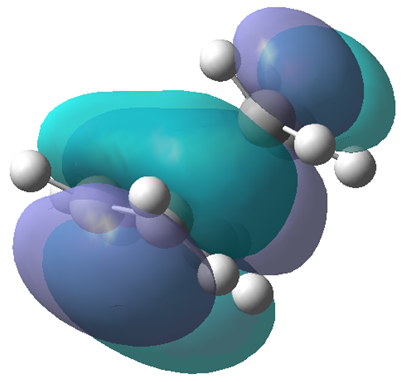
The first transition state optimisation gave a C-C terminal bond length of 2.02Å, whilst the second method also gave 2.02Å. Though both methods gave the same result the later required much less computing time. The chair transition state structure was then re-optimised at a higher level of theory B3LYP/6-31G*. The imaginary vibration at -817.97cm-1 of the transition state looks at follows:
Looking at the vibration it is clear that it corresponds to the reaction pathway.
Optimisation of the boat transition state used the QST2 method. The resulting structure was then re-optimised at a higher level of theory B3LYP/6-31G*. Using the energy of the starting conformer the activation energies can be determined.
| Transition State | Chair | Boat | ||
|---|---|---|---|---|
| Theory | HF/3-21G | B3LYP/6-31G* | HF/3-21G | B3LYP/6-31G* |
| Energy/kcalmol-1 | -145343.32 | -147186.73 | -145332.95 | -147178.01 |
| C-C Terminal Bond Length/Å | 2.02 | 1.96 | 2.14 | 2.21 |
| C-H Bond Length/Å | 1.08 | 1.09 | 1.07 | 1.09 |
| Activation Energy / kcalmol-1 | 45.94181 | 34.34645 | 56.30834 | 43.06331 |
The activation energy is very different when comparing the methods employed. The B3LYP/6-31G* basis set is much more accurate than that of HF/3-21G and hence shows a lower activation. Comparing the boat and transition states the boat transition state has a higher activation energy.
Intermediate Reaction Coordinate (IRC)
Using the IRC method the different stages of an optimisation can be observed. Initially using HF/3-21G the chair transition state was observed through 50 cycles. However the optimisation was not complete, the number of cycles was then increased to 200. After 200 cycles had completed.
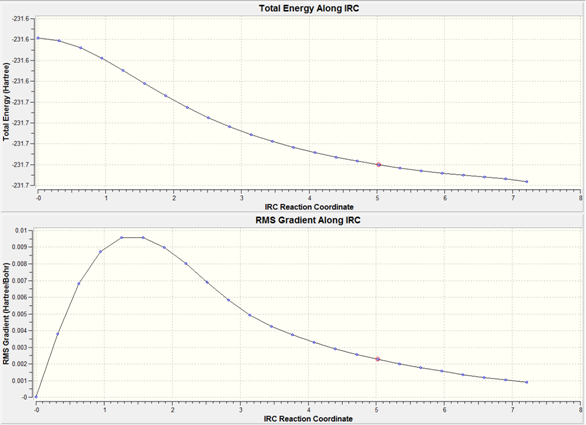
50 Cycles
After 50 cycles it is clear that a minimum has not been reached the energy has not yet plateaued.
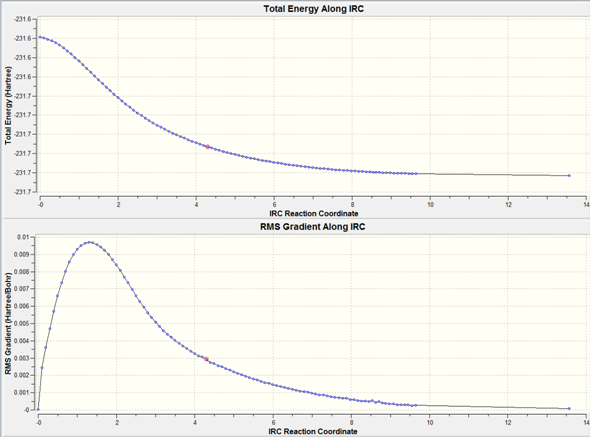
200 Cycles
After 200 cycles the energy has plateaued this indicates that a minimum has been reached. Increasing the number of cycles allows for the optimisation to go to completion.
The Diels Alder Cycloaddition
Cis-butadiene + ethene
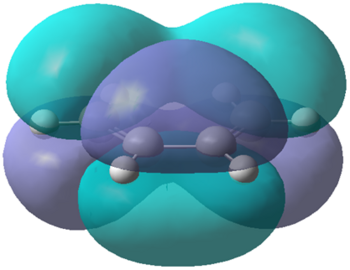
Cis-butadiene was defined in gausview and optimised using the semi-empirical AM1 method. The HOMO and LUMO of cis-butadiene was then examined to determine symmetry, with both the HOMO being a and the LUMO being s. This can be seen more clearly in the pictures below. HOMO is displayed on the left, whilst the LUMO is displayed on the right.
The transition state is shown below:
Pentahelicene |
The partial C-C bond has a bond length of 2.12Å. Typical C-C bond lengths for sp2 carbons are around 1.34Å[2] giving a van der waals radius of 0.67Å, whilst for sp3 carbons are around 1.54Å[2] The partial bond is longer than that expected for a single bong an therefore shows this is indeed a transition state.This is dually supported by an elongation of the double bonds in the transition state to 1.38Å.
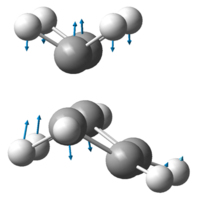
The vibration corresponding to the reaction pathway is at -956.08cm-1 and is visualised below:
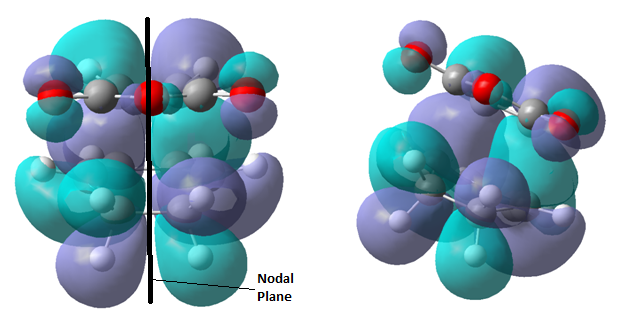
The vibration demonstrates that the formation of the two new σ-bonds is synchronous. Analysis of the lowest vibrational frequency at 147.07 shows a twisting about the partial C-C bonds, it shows no distortion in the bond length between the reacting carbon centres indicating it is not related to the reaction pathway. The transition state HOMO and LUMO are displayed respectively below.
Examining the HOMO it can be seen that is asymmetric therefore can be labelled a. By inspection it is clear that the HOMO(a) of the transition state is formed from the HOMO(a) of cis-butadiene and the LUMO(a) of ethene. The LUMO(s) of the transition state is formed from the LUMO(s) of cis-butadiene and the HOMO(s) of ethene. The reaction is allowed as symmetry is not broken.
Malaeic anhydride + cyclohexa-1,3-diene
The endo and exo transition states for the reaction between maleic anhydride and cyclohexa-1,3-diene were defined in gaussview and optimised using the semi-empirical AM1 method.
| Endo | Exo | ||
|---|---|---|---|
| Energy / a.u. | -0.05159 | -0.05050 | |
| Energy / kcalmol-1 | -32.37589 | -31.69122 | |
| Bond lengths | Partial C-C / Å | 2.16 | 2.17 |
| Anhydride C=C / Å | 1.41 | 1.41 | |
| Cyclohexa-1,3-diene C=C / Å | 1.39 | 1.39 | |
| Through Space Distance / Å | 2.89 | 2.94 | |
Examining the relative energies the exo-transition state is higher in energy and is therefore less stable than the endo-transition state. thought the trough space distance is less for the endo-transition state and would indicate greater steric interactions the reaction does not occur on the same face as the bridging. In the case of the exo-transition structure it does occur on the same face as the bridging and is therefore subject to greater steric hindrance. The HOMO of the endo-transition state looks as follows:
The HOMO of the exo-transition state is as follows:
Looking at the HOMO's for both transition states it becomes clear there is no secondary orbital overlap, this indicates that the stability of the transition state is reliant on sterics.
References
- ↑ Iteractions between non-bonded atoms, and the structure of cis-decalin: DOI:doi:10.1039/JR9480000340
- ↑ 2.0 2.1 Conformational investigation of gaseous 1,5-hexadiene by electron diffraction and molecular mechanics: DOI:doi:10.1016/0022-2860(94)09007-C
Output Files
Anti1 Media:anti11clm08.txt Gauche 1 Media:gauche1clm08.txt Gauche 2 Media:gauche2clm08.txt
Chair HF/3-21G Media:chairopt1clm08.txt Chair B3LYP/6-31G* Media:chairopt2clm08.txt
Boat HF/3-21G Media:boatopt1.txt Boat B3LYP/6-31G* Media:boatopt2.txt
Cis-butadiene + ethene Media:cbeclm08.txt Cis-butadiene + ethene freq analysis Media:cbefreqclm08.txt
Malaeic anhydride + cyclohexa-1,3-diene ENDO Media:macdendoclm08.txt Malaeic anhydride + cyclohexa-1,3-diene EXO Media:macdexoclm08.txt