Rep:Mod3:shl108
Cope Rearrangement
Optimizing the Reactants and Products
Optimisation with HF/3-21G
HF/3-21G level of Gaussian calulation is used to optimise the 1,5-hexadiene in this section.
|
|
| ||||||||||
| File Type | .chk | .chk | .chk | |||||||||
| Calculation Type | FOPT | FOPT | FOPT | |||||||||
| Calculation Method | RHF | RHF | RHF | |||||||||
| Basis Set | 3-21G | 3-21G | 3-21G | |||||||||
| Final Energy / a.u. | -231.69260236 | -231.69153034 | -231.69266122 | |||||||||
| RMS Gradient Norm / a.u. | 0.00001239 | 0.00000795 | 0.00000413 | |||||||||
| Dipole Moment / Debye | 0.20 | 0.13 | 0.34 | |||||||||
| Point Group | C2 | C2 | C1 | |||||||||
| Log file | File:Anticonformer1.LOG | File:GaucheConformerA.LOG | File:GAUCHE3.LOG | |||||||||
| conformer identified from Appendix 1 | anti1 | gauche4 | gauche3 |
Both structures have C2 symmetry. My hypothesis is that gauche conformation is expected to be less stable than the anti conformation due to steric effect. In this case, gauche 4, indeed, has a lower energy than anti 2.However, gauche 3 is the most stable conformation of 1,5-hexandiene. This can be explained by stereoelectronic which is due to the CH-π interaction. The favourable donation of electron density from the πC=C orbital of the C=C double bond into the σ*C-H orbital of the adjacent vinyl proton has lowered the overall energy of the molecule.
Optimisation of Anti2-Conformer with DFT/B3LYP/6-31G(d)
| |||
| File Type | .chk | .chk | |
| Calculation Type | FOPT | FOPT | |
| Calculation Method | RHF | RB3LYP | |
| Basis Set | 3-21G | 6-31G(D) | |
| Final Energy / a.u. | -231.69253506 | -234.61170276 | |
| RMS Gradient Norm / a.u. | 0.00006411 | 0.00003510 | |
| Dipole Moment / Debye | 0.00 | 0.00 | |
| Point Group | Ci | Ci | |
| C1-C4 dihedral angle / ° | 114.7 | 118.5 | |
| Log file | File:Anticonformer2.LOG | File:Anticonformer2DFT.LOG |
The final energies of this molecule calculated by HF/3-21G and B3LYP/6-31G* give two different values, -231.69253506 and -234.61170276 a.u. respectively. Although the dipole moment and point group do not have any changes, the C1-C4 dihedral angles are obtained differently by HF/3-21G and B3LYP/6-31G* which are 114.7° and 119.5° respectively.
| 298.5 K | 0K |
|---|---|
 |

|
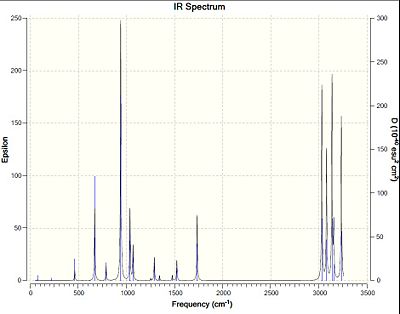
Comparison of Energies at 0K and 298K
The sum of electronic and zero-point energies, the sum of electronic and thermal energies, the sum of electronic and thermal enthalpies and the sum of electronic and thermal free energies at 298.15K and 0K are calculated and shown in the table below.
| Energies | 298.15K | 0K |
|---|---|---|
| Sum of electronic and zero-point Energies (Hartree/Particle) | -234.469211 | -234.468774 |
| Sum of electronic and thermal Energies (Hartree/Particle) | -234.461854 | -234.461428 |
| Sum of electronic and thermal Enthalpies (Hartree/Particle) | -234.460910 | -234.460483 |
| Sum of electronic and thermal Free Energies (Hartree/Particle) | -234.500825 | -234.500376 |
| Output file | File:DFToptFREQUENCY.LOG | File:FREQUENCY2.LOG |
As the temperature increases, the molecule has more energy to access more vibrational state which give more vibrational modes. Thermal energy is temperature dependent, so at high temperature, the contribution of thermal energy increases.
Optimizing the "Chair" and "Boat" Transition Structures
The transition states of Cope rearrangement of 1,5-hexadiene are proposed as below:
For route b, it is the most unlikely pathway. This is because the dissociation energy of the C-C bonds is much higher then the activation energy and there is no cross product produced experimentally. For route c, it has the same problem with high energy TS compare with the activation barrier. Therefore, it is unliky to happen as well.
Optimisation of Chair Transition State
Comparison of TS Optimisation Methods
| CH2CHCH2 | 
|
Checkpoint file | File:CB1.LOG |
| Berry TS | Freeze Coordinate | Redundant Coord Editor | |
|---|---|---|---|
| Energy / Hartree | -231.61932246 | -231.61518364 | -231.61932201 |
| Bond breaking/forming length / Å | 2.02 | 2.20 | 2.02 |
| Imaginary Frequency / cm-1 | -818 | N/A | -818 |
| chair transition state | [1] | N/A | [2] |
| Checkpoint file | File:BerryTSChair.fchk | File:FreezeChairCoordinate.fchk | File:DerivativeCoordinate.fchk |
Two different methods give the same results for the energy of the transition state. Both methods give the same bond breaking/forming length,at 2.02 Å. Vibration at -818 cm-1 shows the bond breaking/forming transition state of this electrocyclic rearrangement. This vibration shows the reaction mechanism is asynchronous, which means one bond breaks and the other forms simultaneously.
Intrinsic Reaction Coordinate
| Energy / Hartree | IRC Path | Gradient | Structure | Files | |
|---|---|---|---|---|---|
| Calculating force constant Once |
-231.68906651 |  |
 |
 |
http://hdl.handle.net/10042/to-5577 |
| Calculating force constant Always |
-231.69164800 |  |
 |
 |
http://hdl.handle.net/10042/to-5576 |
The HF/3-21G optimised chair TS is further investigated with Intrinsic Reaction Coordinate (IRC) calculation. The aim is to simulate the transformation from the chair TS down the potential energy surface to the final product of the reaction.
At first,the IRC calculation was performed with 50 points on the IRC pathway with computating the force constants only once. The result of the calculation showed no bond formation process. This can be explained that the calculation was incomplete, as a 1,5-hexadiene structure was expected. In addition, analysis of the IRC pathway showed that points on the pathway do not reach an asymptotethe which indicaten the minimum of the potential curve has not been reached. Th calculation was repeated with the same number of points on the IRC pathway with computating the force constants at every step. This allows the IRC calculation to determine if it is moving along the minimum energy pathway down the potential energy surface, and make changes to the pathway when necessary. As a result, a conformer of 1,5-hexadiene was observed, and the IRC pathway has reached the minimum of the potential curve.
The optimised chair transition structure connects to the gauche2 conformer.
Optimisation of Boat Transition State
| QST2 | |
|---|---|
| Energy / Hartree | -231.60280222 |
| Bond forming length / Å | 2.14 |
| Imaginary Frequency / cm-1 | -840 |
| Vibration | [3] |
| Log file | File:BOATOPTQST2.LOG |
QST2 is more reliable method that Berry TS. With QST2, the structures of reactant and product are taken as consideration in the calculation.Hammond's postulate[4] can be applied to a more accurate prediction of the TS. In Computational Chemistry, if more information is provided in the calculation, a more accurate result should be obtained. The imaginary frequency, -840 cm-1, shows that a transition state was found. The vibration indicates that the bond dissociation/formation is asynchronous, a stepwise mechanism.
Activation Energies of Chair and Boat Structures
Summary of energies (in hartree)
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||
| Chair TS | -231.619322 | -231.466700 | -231.461341 | -234.556983 | -234.414929 | -234.409008 |
| Boat TS | -231.602802 | -231.450928 | -231.445309 | -234.543093 | -234.402342 | -234.396008 |
| Reactant (anti2) | -231.692535 | -231.539542 | -231.532571 | -234.611702 | -234.468774 | -234.461854 |
*1 hartree = 627.509 kcal/mol
Summary of activation energies (in kcal/mol)
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.71 | 44.70 | 33.79 | 33.16 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.61 | 54.76 | 41.69 | 41.69 | 44.7 ± 2.0 |
| HF (298.15K) | HF (0K) | DFT (298.15) | DFT (0K) | |
|---|---|---|---|---|
| Chair | File:BerryTSChair.out | File:BerryTSchair0K.out | File:DFTChair.out | File:DFTChair0K.out |
| Boat | File:BOATOPTQST2.LOG | File:Boatopt0K.out | File:BoatoptDFT.out | File:BoatoptDFT0K.out |
| Anti2 | File:DFToptFREQUENCY.LOG | File:FREQUENCY2.LOG | File:Anti2HFopt.out | File:Anti2HFopt0K.out |
The calculated activation energies agrees with the experimental values for both chair and boat structures.
Diels Alder Cycloaddition
For both optimisations of the transition states of cycloaddition, QST2/AM1 method is used to perform the calculation.
Optimisation of cis-butadiene and ethene
| Butadiene | Ethene | ||||
|---|---|---|---|---|---|
| MO | Representation | Symmetry | MO | Representation | Symmetry |
| HOMO | 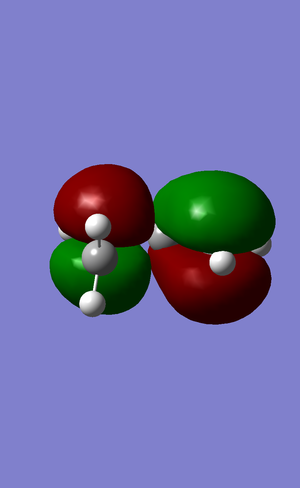 |
Antisymmetric | HOMO |  |
Symmetric |
| LUMO | 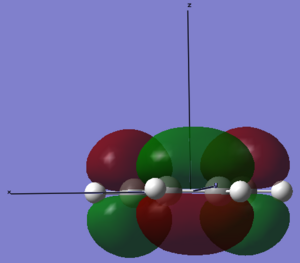 |
Symmetric | LUMO |  |
AntiSymmetric |
| Files | Butadiene[5] | Ethene [6] | |||
The LUMO of cis-butadiene can be reflected in the plane of symmetry of the molecule. Therefore, it is symmetric. For the HOMO of cis-butadiene, the phases are inverted while the orbital is reflected in the plane of symmetry, so it is anti-symmetric. The symmetry of the orbitals can give informtaion of which orbitals are going to react and how the stereoelectronic control will be. The HOMO of ethene is symmetric with respect to the plane of symmetry and the LUMO is anti-symmetric. In quantum theory, only the orbitals with the same symmetry can interact. Therefore, the HOMO of cis-butadiene will interact with the LUMO of ethene, and the LUMO of cis-butadiene will interact with the HOMO of ethene.
This can be proved in the transition state calculation.
Computation of the Transition State geometry for the prototype reaction
| QST2 | ||
|---|---|---|
| Bond forming length / Å | 2.12 | |
| Vibrational frequency / cm-1 | -956 | 147 |
| Vibration |  |
|
| Sum of electronic and zero-point Energies/a.u. | 0.253274 | |
| Sum of electronic and thermal Energies/a.u. | 0.259451 | |
| Sum of electronic and thermal Enthalpies/a.u. | 0.260395 | |
| Sum of electronic and thermal Free Energies/a.u. | 0.224014 | |
| Structure | 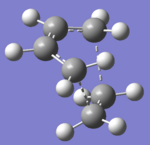
| |
| Files | [7] |
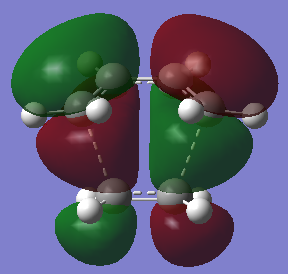 |
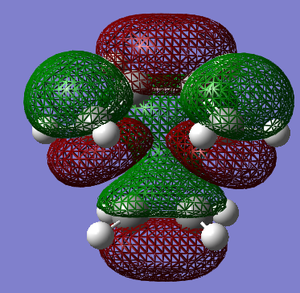 |
The bond breaking/forming length in the TS is 2.12 Å. The typical sp3 C-C bond is 1.53 Å, and sp2 C=C bond is 1.34 Å. Van de Waals Radius of carbon is 1.70 Å.[2] The bond breaking/forming length is shorter than the Van de Waals interaction betweent two carbon atoms which is 3.40Å. However, it is longer than sp3 and sp2 C-C bond length. This means that the carbon atoms are approaching each other within the Van de Waals radii region for bond formation, but the bonds are partially formed and broken. This fits with the transition state theory. According to the quantum theory,the symmetry of the molecular orbital formed form the overlap of two atomic orbitals, have to be the same as that of the initial orbitals. In addition, only filled orbitals can overlap with empty orbitals and vice versa. This can be explained by Pauli exclusion principle[8] and Hund's rule[9]. The HOMO is antisymmetric, whereas the LUMO is symmetric. The negative frequency at -956 cm-1 which indicates formation of the two bonds is synchronous. The lowest positive frequency at 147 cm-1 is the rocking vibration which indicates the motion of the viration is asynchronous.
The regioselectivity of the Diels Alder Reaction
| Endo | Exo | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Energy/ a.u. | -0.05159413 | -0.05050324 | ||||||
| Dipole Moment/ Debye | 6.17 | 5.57 | ||||||
| Bond breaking/forming length/ Å | 2.16 | 2.17 | ||||||
| Imaginary Frequency / cm-1 | -805 | -812 | ||||||
| Structure |
|
| ||||||
| Files | [10] | [11] |
The endo transition state have a more negative energy than exo transition state. It means the activation barrier of obtaining endo transition state is lower. If the final product is selective to the endo product, the reaction will be controlled kinetically.
The C-C bond breaking/forming length is 2.16 Å in the endo transition state, and 2.17 Å in the exo transition state. Endo transition state has a shorter C-C bond breaking/forming length than exo transition state which indicates the stronger interaction and more likely to form bond. In addition, the distance between the carbon atom of the carbonyl group in the maleic anhydride and the non-reacting carbon atom of the C=C double bond on the cyclo-1,3-hexadiene. In the endo transition state, it is 2.89 Å, while in the exo transition state, it is 3.78 Å. This distance shows the secondary orbital overlap between the πC-C orbital of cyclo-1,3-hexadiene and π*C=O orbital of maleic anhydride, which can overall stabilise the transition state. The shorter distance in the endo transition state than the exo transition state indicates that secondary orbital overlap only occur in endo transition state.
| Parameter | Endo / Angstroms | Exo / Angstroms |
|---|---|---|
| Length of new/forming C-C sigma bonds | 2.16 | 2.17 |
| Distance between C=O group and opposite CH2CH2 group | 2.89 | 3.78 |
| Distance between the anhydride C=C and the diene C=C | 2.71 | 2.64 |
| Distance between the two C=O groups | 2.28 | 2.28 |