Rep:Mod3:jcl08m3
Computational lab Module 3
Cope Rearrangement

The Cope rearrangment[1] reaction is a [3,3]-sigmatropic rearrangment reaction, which occurs via a single concerted cyclic transition state.
Optimizing the Reactants and Products
Part (a)
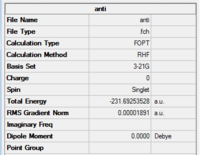
For the anti conformation, the point group is found to be Ci. By comparing the energies and symmetry of the molecule to data in Appendix 1, the optimised structure is identified to be anti2.
Part (b)
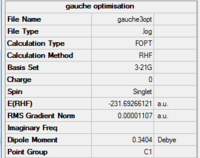
For the gauche conformation, the point group is found to be C1. By comparing the energies and symmetry of the molecule to data in Appendix 1, the optimised structure is identified to be gauche3.
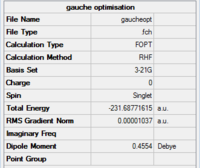
Making comparison between the structure of anti2 and gauche3, we would expect gauche1 to be the less stable conformer as there are larger steric hindrance, thus repulsions within the structure. Yet this is proven to be wrong from the calculation, where the electronic energy is lower for gauche3 (-231.69266121 a.u.) than anti2 (-231.69253528 a.u.). This result can be explained by the stereoelectronic effect, where the п orbital of the C=C interacts with the σ*C-H orbital of the adjacent vinyl proton and stabilse the gauche conformation.
Part (c)&(d)
As mentioned above, the energy of the conformer is a balance of both steric and stereoelectronic factors, where the stereoelectronic factor has a larger contribution to stablility. Therefore, we would expect gauche3 to be the most stable structure. This is proven by producing the other gauche structure for comparison.

 |
 |
| Conformer | Energy / a.u. | Point Group | file on D-space |
|---|---|---|---|
| anti2 | -231.692535 | Ci | http://hdl.handle.net/10042/to-5612 |
| gauche3 | -231.692661 | C1 | http://hdl.handle.net/10042/to-5614 |
| gauche5 | -231.689616 | C1 | http://hdl.handle.net/10042/to-5616 |
| gauche4 | -231.691530 | C2 | http://hdl.handle.net/10042/to-5615 |
| gauche1 | -231.687716 | C2 | http://hdl.handle.net/10042/to-5613 |
Comparing the different conformation, gauche3 has the lowest electronic energy of -231.692661.
Part (e)
The anti2 conformer is optimised in previous section, the data are provided above.
Part (f)
The anti2 conformer is re-optimised using a more accurate basis set (DFT/B3LYP/6-31G*). Comparison is made between the two sets of result in the table below.
| molecule |
|
| ||||||
| Basis set | HF/3-21G | B3LYP/6-31G* | ||||||
| File Type | .log | .log | ||||||
| Calculation Type | FOPT | FOPT | ||||||
| Calculation Method | RHF | RHF | ||||||
| Final Energy / a.u. | -231.692535 | -234.611703 | ||||||
| RMS Gradient Norm / a.u. | 0.00001891 | 0.00001326 | ||||||
| Dipole Moment / Debye | 0.0000 | 0.0000 | ||||||
| Point Group | C1 | C1 | ||||||
| Output file | http://hdl.handle.net/10042/to-5612 | http://hdl.handle.net/10042/to-5617 |
Most data remained the same from a better basis set theory. However, the final energy of the molecule lowered by around 3 a.u. when the anti conformer is re-optimised, indicating that the structure is further optimised to a lower energry state.
| Basis set used | Bond length/ Angstroms and Torsional angle / ° | |||||
|---|---|---|---|---|---|---|
| C1-C2,C5-C6 | C2-C3,C4-C5 | C3-C4 | C2-C3-C4-C5 | C1-C2-C3-C4 | C6-C5-C4-C3 | |
| HF 3-21G | 1.32 | 1.51 | 1.55 | 180.0 | 114.7 | 114.7 |
| B3LYP 6-31G* | 1.33 | 1.50 | 1.55 | 180.0 | 118.5 | 118.5 |
In terms of geometry, the molecule has not change significantly, the torsional angle increased by around 4 degrees, while the bond length shifted by around 0.01 angstroms. Making comparison with the literature values( lit: C=C is 1.34 angstroms and C-C is 1.54 angstrom), the simulated bond lengths are in agreement with the value.
Part (g)
A frequency analysis is carried out on the re-optimised anti2 structure. There are no imaginary vibration frequency in the result and the IR spectrum is shown below:
 |
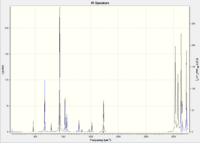 |
No imaginary vibration indicating that the structure optimised at the previous step is at its minimum.
An optimisation at 0K was also carried out, the thermochemistry data of the two frequency analysis are shown in the table below:
| Table 4 - Thermochemistry data from frequency analysis of 6-31G(d)-optimized 1,5-hexadiene | ||
|---|---|---|
| Analysis at 298.15K | Analysis at 0K | |
| Final energies / Eh | -234.611703 a.u. | -234.611703 a.u. |
| Sum of electronic and zero-point energies / Eh | -234.469212 a.u. | -234.468774 a.u. |
| Sum of electronic and thermal energies / Eh | -234.461856 a.u. | -234.461429 a.u. |
| Sum of electronic and thermal enthalpies / Eh | -234.460912 a.u. | -234.460485 a.u. |
| Sum of electronic and free energies / Eh | -234.500821 a.u. | -234.500371 a.u. |
| Output file | http://hdl.handle.net/10042/to-5619 | http://hdl.handle.net/10042/to-5620 |
The (electronic)final enegry at both temperature remained the same, but the other sums increase in energy as temperature decrease to 0K.
Optimizing the "Chair" and "Boat" Transition Structures
The transition state of the Coper rearrangement can be adopt either a chair or a boat configurations. In this section, we would analysis the different between the two ts in further details.
Part (a)
 |
An allyl fragment (CH2CHCH2) is optimised using the HF/3-21G level of theory. Using this fragment, a guess transition structure is produced.
Part (b)&(c)&(d)
Optimisation of the guess transition state structure (TS Berny) was carried out using HF/3-21G basis set, followed by an frequency analysis. The result showed a imaginary vibration at -817.95 [[1]]
The same calculation with ModRedundant Minimisation which frozen C-C lengths, at 2.20 Å, for the C atoms involved in bond breaking/forming was then carried out. There is a imaginary vibration at -818.01 [[2]]
The data of the two calculation are listed below:
| Force Constant | Redundant Coordinate | |||||||
| Structure |
|
| ||||||
| C-C bond length within allyl fragments (Å) | 2.02 | 2.02 | ||||||
| Final Energy (a.u.) | -231.61932247 | -231.61932204 | ||||||
| RMS Gradient (a.u.) | 0.00000829 | 0.00005617 | ||||||
| Spin | Singlet | Singlet | ||||||
| Charge | 0 | 0 | ||||||
| Dipole (D) | 0.0000 | 0.0000 | ||||||
| Point Group | C2h | C2h | ||||||
| Frequency of Imaginary Vibration (cm-1) | -818 | -818 | ||||||
| output file on D-space | http://hdl.handle.net/10042/to-5627 | http://hdl.handle.net/10042/to-5626 |
Using redundant coordinate method, we obtained a lower final energy ie. a better optimised structure. the point group, dipole and the imaginary vibration remained the same.
Part (e)
For the optimisation of the boat transition state, we are using a different method known as QST2. QST2 involve the coordinates of the starting product and reactants, and from that deduce the transition state.
Opt+Freq job was run with a basis set of HF/3-21G. However, the first job failed to give the right transition state geometry:
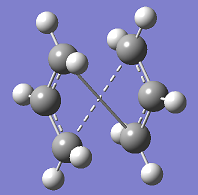
Output file on D-space: http://hdl.handle.net/10042/to-5636
This is due to the readily rotation of the central C-C bond. The starting structure is too different from the expecting product. Therefore,for the second time, the bond angles and torsion angles are adjusted before the optimisation, and the QST2 successful produce the boat transition state:
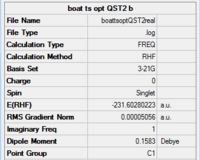
|
There is an imaginary bond breaking vibration at -840.08, therefore this is a asynchronous bond formation.
Part (f)
Despite the fact that we obtained the transition state geometry of both chair and boat, we cannot fom that deduce the geometry of the final product. Intrinsic Reaction Coordinate (IRC) calculation is therefore used to model the transformation from chair transition state down the potential energy surface to the final product of the reaction. The method was applied twice to the chair ts. The first time having it to calculate the force constants once, and the second time at every single steps. The latter method provide a better result, this can be demonstrated by the IRC energy curve where the latter method reaches closer to the minimum. This is also reflected by the energy of the structure, where the latter method is around 0.026 a.u. lower. From the energy and the point group of the IRC final structure, we can deduce that the chair ts leads to the formation of the Gauche2 conformer.
| Calculate Force Constants Once | Calculate Force Constants always | |
|---|---|---|
| Points along IRC | 20 | 47 |
| IRC energy pathway & RMS gradient | 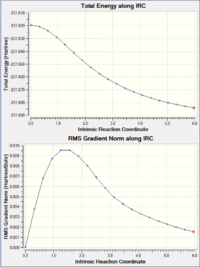 |
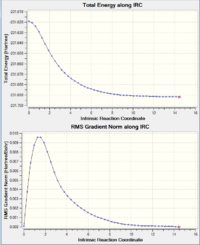
|
| Final structure of IRC |  |
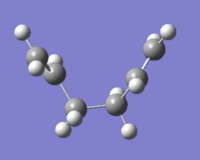
|
| Energy / a.u. | -231.68409949 | -231.69166411 |
| point group | C2 | C2 |
| out put file on D-space | http://hdl.handle.net/10042/to-5624 | http://hdl.handle.net/10042/to-5625 |
Part (g)
Optimisation and frequency analysis were carried out to both boat and chair ts, at different temperature(298.15k and 0K) and usinfg different basis set (HF/3-21G and B3LYP/6-31G*). The thermochemistry data of the result are shown below, in comparison with the anti2 values:
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy / a.u. | Sum of Electronic and zero point energies at 0K / a.u. | Sum of Electronic and thermal enthalpies at 298.15K / a.u. | Electronic energy / a.u. | Sum of Electronic and zero point energies at 0K / a.u. | Sum of Electronic and thermal energies at 298.15K / a.u. | |
| Chair Transition State | -231.619322 | -231.466224 | -231.461341 | -234.556983 | -234.414485 | -234.409008 |
| Boat Transition State | -231.602802 | -231.450456 | -231.445301 | -234.543093 | -234.401903 | -234.396008 |
| Reactant anti2 | -231.692535 | -231.539069 | -231.532565 | -234.611703 | -234.468774 | -234.461856 |
From the data above, the activation energy of the boat and chair transition reaction are calculated:
| HF/3-21G | B3LYP/6-31G* | Experimental Data | |||
|---|---|---|---|---|---|
| at 0K | at 298.15K | at 0K | at 298.15K | at 0K | |
| ΔE Chair | 45.71 | 44.69 | 34.07 | 33.16 | 33.5 +/- 0.05 |
| ΔE Boat | 55.61 | 54.76 | 41.96 | 41.32 | 44.7 +/- 2.0 |
The activation energies match well with literature values at 0K. The activation energy for chair ts is lower than the Boat ts, suggesting that the reaction via chair ts is a preferred pathway.
The Diels Alder Cycloaddition

In this section, we are looking at the diels alder cycloaddition between butadiene and ethylene. The molecular orbital are simulated for both the reactants and the transition state.
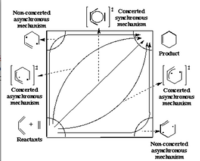
The reaction transition state are consider to be either synchronous or asynchronous. It can either be concerted, bond breaking followed by bond formation, or vice versa. This is shown by the diagram on the right.
part (i)
The structures of both ethylene and butadiene are optimised and their molecular orbital are simulated using semi-empirical AM1 basis set.
Looking at the molecular orbital, we would expect interaction to occur between the HOMO of butadiene and the LUMO of the ethylene. The same assumption is made by looking at the energy of the orbital(ethyleneLUMO-butadieneHOMO = 0.05284-(-0.34380)= 0.39664 and butadieneLUMO-ethyleneHOMO = 0.01707-(-0.38775) = 0.40482), where the orbitals of ethylene LUMO and butadiene HOMO are closer in energy. This agrees with our knowledge that the diene is acting like an electron acceptor, while the ethylene being the donator.
Part (ii)
The transition state of the Diels Alder cycloaddition are produced using the QST2 method. QST2 was chosen over the TS(Berny) method as QST2 requires less step while providing a higher success rate in obtaining the correct transition structure.
 |
|
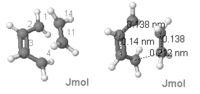 |
The bond length of the transition state are listed below:
| Bond | Bond length / Å |
|---|---|
| C1-C2 | 1.38 |
| C2-C3 | 1.40 |
| C3-C4 | 1.38 |
| C4-C11 Bond forming | 2.12 |
| C11-C14 Ethene bond | 1.38 |
| C14-C1 Bond forming | 2.12 |
Typical values of the Csp3-Csp3 bond length is 1.53 Å, and the unconjugated Csp2-Csp2 bond length is 1.48 Å[1]. These are slightly different from the calculated result.
The literature Van der Waals radius of a carbon is 1.70Å[2].. The distance between the bond forming carbon is 2.12Å, indicating that the atoms are approaching within their Van der Waals radii. However, 2.12Å is still longer than a normal C-C single bond,and the bond is not fully formed in the transition state.
 |
 |
 |
 |
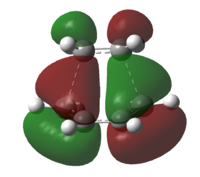
The imaginary vibration at -956cm-1 indicates the bond formation vibration, and the motion of the vibration is synchronous. This suggest that the Diels-Alder reaction is a concerted reaction. The rocking motion of the lowest positive frequency vibration at 147cm-1 is asynchronous.
Looking at the orbital of the transition state, we can identify that the HOMO is correspond to the interaction between the LUMO of ethylene and HOMO of butadiene, whereas the LUMo of the transition state correspond to the interaction between the HOMO of ethylene and the LUMO of butadiene.
A better basis set (DFT/B3LYP/6-31G* method) was used to re-optimise the transition structure.
The two set of data is shown below:
| Properties | Semi-empirical/AM1 | DFT/B3LYP/6-31G(d) |
|---|---|---|
| Electronic Energy / a.u. | 0.111655 | -234.543896 |
| Sum of Electronic and Zero-Point Energies / a.u. | 0.253275 | -234.403321 |
| Sum of Electronic and Thermal Energies / a.u. | 0.259452 | -234.396903 |
| Sum of Electronic and Thermal Enthalpies / a.u. | 0.260397 | -234.395959 |
| Sum of Electronic and Thermal Free Energies / a.u. | 0.224014 | -234.432887 |
| Imaginary Frequency / cm-1 | -956 | -525 |
| C-C Bond Forming distance / Å | 2.12 | 2.27 |
| Output file on D-space | http://hdl.handle.net/10042/to-5637 | http://hdl.handle.net/10042/to-5638 |
part (iii)
In this section we study the reaction between Cyclohexa-1,3-diene and maleic anhydride. The reaction can result in two different products, it can either be an exo form or an endo form. The molecular orbitals and transition states structure are simulated below for further investigation energies of the reaction.
| Structure | HOMO | LUMO | Energy / a.u. | ||||
| maleicanhydride |
|
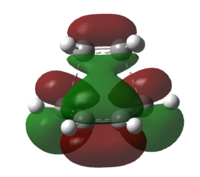
 |
 |
HOMO:-0.44185 LUMO:-0.05952 | |||
| Cyclohex-1,3-diene |
|
 |
 |
HOMO:-0.32074 LUMO:0.01692 |
| Exo TS AM1 |
Endo TS AM1 | |||||||
| transition state |
|
| ||||||
| Energy / Hartree a.u. | -0.050503 | -0.051594 | ||||||
| Sum of electronic and thermal energies / Hartree |
0.14479 | 0.14359 | ||||||
| Imaginary Frequency / cm-1 | -811.47 | -805.32 | ||||||
| Output file on D-space | http://hdl.handle.net/10042/to-5640 | http://hdl.handle.net/10042/to-5639 |
Comparing the transition state of both exo and endo structure, the endo is lower in energy. As the reaction is a irreverisble, thus kinetic control dominates, endo structure would therefore be the primary product of the reaction between maleicanhydride and cyclohex-1,3-diene. Exo is more strained as steric hindrance between the oxygen from the maleicanhydride and the hydrogen on the sp3 carbon is larger than in endo, where the sp3 carbons are replaced by sp2, one hydrogen less.
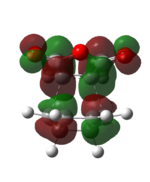
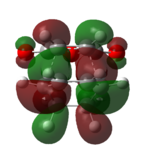
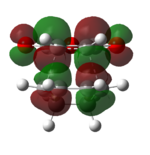
| Endo TS | Exo TS | ||
|---|---|---|---|
| HOMO (-0.34506 a.u.) | LUMO (-0.03570 a.u.) | HOMO (-0.34275 a.u.) | LUMO (-0.04044 a.u.) |
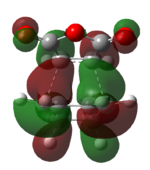
|
|
|
|
| Anti-symmetric | Anti-symmetric | Anti-symmetric | Anti-symmetric |
Both the HOMO and the LUMO of the endo and exo transition state are antisymmetric. If we compare the HOMO and LUMO of the transition states to the reactants'. We can identify that the reaction only involve the interaction between the Cyclohex-1,3-diene HOMO and maleicanhydride LUMO.
Secondary orbital interactions is only observed in the endo HOMO, the π*C=O orbital are in the same phase as the π conjugated system in the cyclo-1,3-hexadiene, thus an interaction between the orbitals at the back of the molecule stabilise the endo transition and giving large overlap between the orbitals of the bond forming carbons, indicating a favourable bonding interaction.[3]
Reference
- ↑ F.H. Allen, O. Kennard, D.G. Watson, L. Brammer, A.G. Orphen, R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1-S19: DOI:10.1039/P298700000S1
- ↑ A. Bondi, J. Phys. Chem., 1964, 68, 441: DOI:10.1021/j100785a001
- ↑ R. Hoffmann, R.B. Woodward, J. Am. Chem. Soc., 1965, 87, 4388: DOI:10.1021/ja00947a033