Rep:Mod3:cathyleephysical
Module 3
Cope Rearrangement Tutorial
In this reaction, a cope rearrangement of 1,5-hexadiene occurs in a [3,3]-sigmatropic shift rearrangement. In this exercise, the study of the transition state will be conducted using Gaussview.
Reactant and Products
| Anti Conformation | Gauche Conformation | Ci Conformation (HF/3-21G) | Ci Conformation (B3LYP/6-31G) | |
|---|---|---|---|---|
| Picture |  |
 |
 |
|
| Point Group | C2 | C2 | Ci | Ci |
| Energy/Hartrees | -231.6926 | -231.6915 | -231.6925 | -234.5597 |
| Ci Conformation | |
|---|---|
| Electronic and Zero-point Energy | -234.4162 |
| Electronic and Thermal Energy | -234.4089 |
| Electronic and Thermal Enthalpy | -234.4079 |
| Electronic and Thermal Free Energy | -234.4478 |
The Anti conformer I obtained corresponds to anti2 and its energy (-231.69260 Hartree) in Appendix 1 . The Gauche conformer corresponds to gauche4 and its energy (-231.69153 Hartree) in Appendix 1 .
It would be expected that the anti conformer would be lower in energy as this conformation makes the two alkene groups be as far away as possible to each other, thus preventing steric hinderance. According to the energies obtained, they seem to follow my predictions with the anti conformer being the most stable.
After three attempts, by turning the hydrogens around, the Ci point group symmetry was obtained. The energy obtained corresponds to the data found in Appendix 1.
After optimising the Ci molecule using a more accurate method and basis set, there was not much change to the geometry of the molecule, the C=C-C angle increased by 0.42 degrees. However, a change in energy was seen, the molecule lowered in energy by 2.87 Hartrees.
The results obtained doing the QST2 calculation using the Ci onformation was that the energy was -231.5189 Hartrees and the only one imaginary frequency was found.
Chair and Boat Transition State
The geometry and vibrations was very similar for both methods used. The imaginary frequency obtained corresponds to what is given in the tutorial. The vibration is shown in the picture above. As you can see the vibrations at the terminal carbons of the allyl group are the opposite to one another. This means the bond breaking/making is asynchronous.
The bond lengths obtained from the two different methods show little difference from each other. however, the bond length is longer in the boat conformation which is expected because there will be some steric hinderance from the allyl groups.
A difference in energy can be seen when the two different basis sets were used (HF/3-21G copared with B3LYP/6-31G). There is a difference of about 3 Hartrees which is expected as the higher basis set is more accurate. As you can see, the more calculations done (opt derivitive -> IRC -> B3LYP/6-31G) the energy lowers at each step.
Diels Alder Cycloaddition Exercies
Cis Butadiene
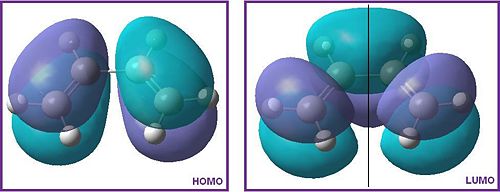
The HOMO molecular orbital is antisymmetric because it only has a centre of inversion. The LUMO MO has σv symmetry label (shown by the black line) suggesting that the molecule is symmetric.
Transition State of a Diels Alder Reaction

The black line shows a σv plane, so the HOMO MO is symmetric. There is one nodal plane on the alkene which corresponds to what looks like a π orbital.
A typical sp2 C=C bond length is 1.350Å, a typical sp3 C-C bond length is 1.537Å [1] and the Van der Waals radius of carbon is 1.70Å[2]. As you can see, in the transition state, the original bonds do not follow its typical bond lengths, instead their bond lengths suggest the bonds that will be the product. The length of of the partially formed bond is near the length of a typical C-C bond so the transition state is at its optimum conformation and distances.
One imaginary vibration was found at -83.512cm-1. The movement of the vibrations are the same suggesting that the formation of the bond is synchronous. The lowest positive frequency can be found at 224.19cm-1 (infrared intensity = 0.1048) and the vibrations look exactly the same as the imaginary frequency.
The LUMO of the cis-butadiene reacted with the LUMO of the ethylene to produce this molecular orbital. Both orbitals are symmetric and hence allowed.
Regioselectivity of a Diels Alder Reaction
| Endo Transition State | Exo Transition State | |
|---|---|---|
| Picture | 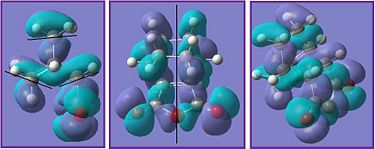 |
 |
| Energy/Hartree | -606.9082 | -570.0622 |
| C-C Steric Distance/Å | 2.974 | 2.962 |
When cyclohexa-1,3-diene reacts with maleic anhydride, the kinetic product dominates so the major product is the endo conformer. This means that the endo transition state should be lower in energy compared to exo which is what the results show ad the difference in the two is relatively large (36.846 Hartrees). This difference in energy is due to the exo form being strained. The endo transition state is stabilised by the secondary orbital effect (see below).
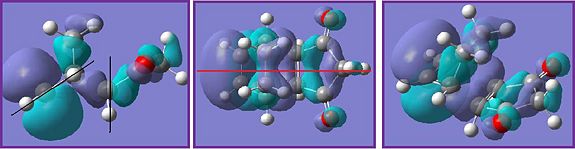
The black lines in the HOMO diagrams represent the nodal planes found in the molecule and the red line represents any symmetry present.In the exo transition state there is one σv plane whilst in the endo transition state there are no symmetry planes but there is cemtre of inversion.
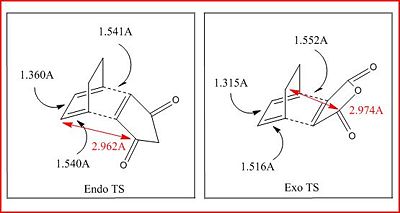
For both transition states it shows that the bond lengths are becoming more like the final product. The C-C bond that is about to be formed have a bond distance similar to a typical C-C bond (1.537Å). The C=C bond on the cyclohexa-1,3-diene has a bond length of around 1.5Å which is too long to be a typical C=C bond (1.350Å) but is more like a C-C bond that it will be. This also applies for the single C-C bond, which will become a C=C bond, the distance is closer to a typical C=C bond than a C-C bond.
There is a small difference between the C-C space orientation (shown in the molecule with a red arrow) between the two conformers. It would be expected that the endo transition state distance should be more than the exo because it is the lower in energy so should be less strained. It endo TS is less constrained however, the distance is only more tha the exo by 0.016Å, however this is probably enough to relieve it from strain. However, the main factor why endo transition is lower in energy is due to the secondary orbital effect[3]. Secondary orbital effect is a stabilising effect where there is overlap between other atoms that are not involved in the bond breaking/forming. There is a strong interaction found in the endo transition state (but not in the exo) thus lowering the energy and making the transition state more stable.
References
- ↑ Bonds in Carbon Compounds http://www.iop.org/EJ/article/0022-3719/19/24/006/jcv19i24p4613.pdf?request-id=1aeae80f-c9b5-41e5-a275-078a1f5b284c
- ↑ Van der Waals Volumes and Radii DOI:110.1021/j100785a001
- ↑ Steric effects vs. secondary orbital overlap in Diels-Alder reactions DOI:10.1021/jo00384a016