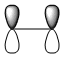Rep:Mod3:Yuko.Isayama3001Ex2
The Diels Alder Cycloaddition
In a Diel-Alder reaction, the π orbitals of the dienophile combine with the π orbitals of the diene to form new σ bonds. The number of π electrons involved determine whether or not the reaction occurs in a concerted stereospecific fashion (allowed) or not (forbidden). Generally the HOMO/LUMO of one reactant interacts with the HOMO/LUMO of the other to form two new bonding/antibonding MOs.
If the dienophile is substituted, with substituents that have π orbitals, they can stabilise the regiochemistry of the reaction by interacting with new double bond that has been formed.
In this section, the transition structures for the Diels-Alder reactions between ethylene and cis-butadiene which is a prototypical reaction, and between that of cyclohexa-1,3-diene and maleic anhydride, where both reactants carry substituents were characterised by the frozen coordinate method, followed by examining the molecular orbitals. For all the calculations the AM1 semi-empirical molecular orbital was used.
Ethylene and Cis-Butadiene
 |
Optimisation and Molecular Orbitals of Cis-Butadiene and Ethylene
Optimisation of cis-butadiene and ethylene based on the AM1 semi-empricial orbital method gave energies of 0.04879719 and 0.02619028 Hartrees respectively, equivalently 30.62068kcalmol-1 and 16.43464kcamol-1.
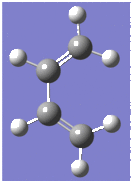 |
 |
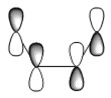
The HOMO and LUMO of each reactants are tabulated with their respective energies and symmetries (the orbitals are classified as symmetric and anti-symmetric with respect to the plane of symmetry shown);
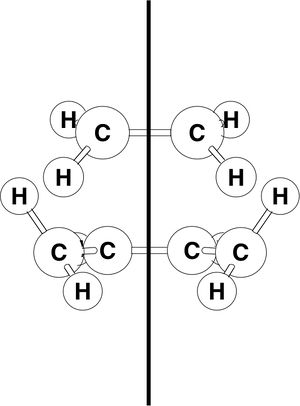 |
| Reactant | Molecular Orbital | Molecular Orbital Image | Energy/Hartrees | Energy/Hartrees (B3LYP/6-31G*) | Symmetry w.r.t the plane | |
|---|---|---|---|---|---|---|
| cis-butadiene | HOMO | -0.34381 | -215.74387 | Anti-symmetric | ||
| LUMO | 0.01707 | 10.71158 | Symmetric | |||
| ethylene | HOMO | -0.38775 | -243.31661 | Symmetric | ||
| LUMO | 0.05283 | 33.15130 | Anti-symmetric | |||
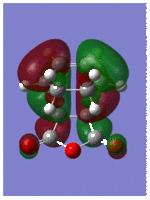
Optmisation and Molecular Orbitals of the Transition Structure
The optimisation of the transition structure was successful which was confirmed by frequency analysis; an imaginary frequency at -956.65cm-1 representing two synchronous bond formations, which is expected for concerted Diels-Alder reaction. In contrast, the lowest positive frequency at 147.21cm-1 corresponds to the 'rocking' motion of ethylene, indicating that it not involved in the reaction pathway to a transiton state.
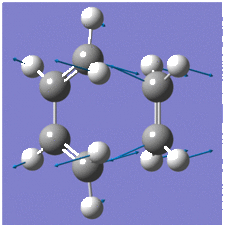 |
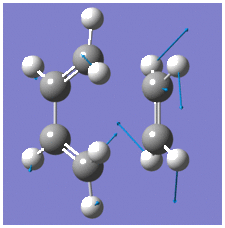 |
The optimised geometry of the transition struture is shown below, including the bond lengths of the partly formed σC-C bonds;
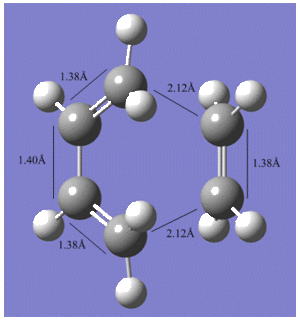 |
Comparison with typical sp3 and sp2 C-C bond lengths, 1.54Å1 and 1.34Å1, indicate that that the C=C bond lengths are in better agreement than the C-C bonds. The partly formed σC-C bond in the transition structure is 2.12Å, which is shorter than twice the van der Waals radius of a carbon atom, 1.71Å, but longer than a typical C-C bond. This suggests that the the van der Waals radii of the terminal carbon atoms are within each other to allow for bond formation, but because it is a transition structure, the bonds have not actually been formed yet.
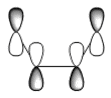
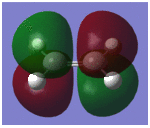
The HOMO and LUMO are shown below with their respective energies;
| Molecular Orbital | Molecular Orbital Image | Energy/Hartrees | Energy/kcalmol-1 | Symmetry w.r.t the plane |
|---|---|---|---|---|
| HOMO | -0.32396 | -203.28782 | Anti-symmetric | |
| LUMO | 0.02319 | 14.55193 | Symmetric |
By comparing the molecular orbitals of the transition structure with the those of reactants, it can be seen that the principal orbital interactions involve the π/π* orbitals of ethylene and the HOMO/LUMO of butadiene as expected. The LUMO of ethylene and HOMO of cis-butadiene are both anti-symmetric with respect to the reflection plane and overlap to form the HOMO of the transition structure, whilst the HOMO of the ethylene and LUMO of the butadiene overlap to form the LUMO of the transition structure because they are both symmetric. Thus, it is evident that orbital symmetry control is exhibited in such concerted reactions which is stated by Conservation of Orbital Symmetry3; transformation of the moelcular orbitals into the products proceed continuously by following the reaction path along which the symmetry of these orbitals remains the same as those of the reactants. Thus, reactions which follow the rule are classified as symmetry-allowed reactions; if the orbitals have different symmetry properties, then no overlap of electron density is possible and the reaction is forbidden.
Additionally, in terms of the molecular orbital energies, the energy difference between the HOMO of the cis-butadiene and LUMO of the ethylene is smaller to form the reactive HOMO (248.87kcalmol-1) than that of the orbitals which are involved in the LUMO of the transition structure(253.83kcalmol-1), thereby implying low kinetic stability.
References
- Fox, MA and JK Whitesell. Organische Chemie. 1994. Spektrum
- Bondi, A. (1964). "Van der Waals Volumes and Radii". J. Phys. Chem. 68 (3): 441–51. DOI:10.1021/j100785a001
- Hoffmann, R. Woodward, R.B. (1968). "Conservation of Orbital Symmetry" Acc. Chem. Res. 1 (1): 17–22 DOI:10.1021/ar50001a003
Cyclohexa-1,3-diene and Maleic Anhydride
Depending upon the orientation in which the dienophile i.e. the maleic anhydride appoaches the diene, two stereoisomer can be formed; the endo-isomer or the exo-isomer. In fact, cyclohexa-1,3-diene 1 undergoes a facile reaction with maleic anhydride 2 to give primarily the endo-adduct. The reaction is said to be kinetically controlled which suggests that the exo-transition state is higher in energy.
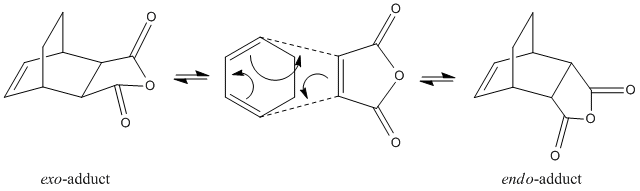 |
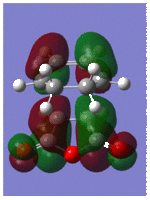
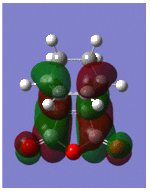
Optimisation and Molecular Orbitals of the Transition Structure
Optimisation was successful and gave the exo-transition structure. In order to locate the endo-transition structure, the maleic anydride was flipped so that the hydrogens were pointing upwards as shown (shown). This time, the TS (Berny) optimisation was applied with the force constants calculated once, which successfully gave the endo-transition structure. Both structures are shown below with their respective energies and imaginary frequencies;
| Exo TS | Endo TS | |
| Orientation of Hs2 | ||
| Structure from side | ||
| Energy/Hartrees | -0.05041981 | -0.05150473 |
| Energy/kcalmol-1 | -31.63888 | -32.31968 |
| Imaginary frequency/cm-1 | -812.17 | -806.49 |
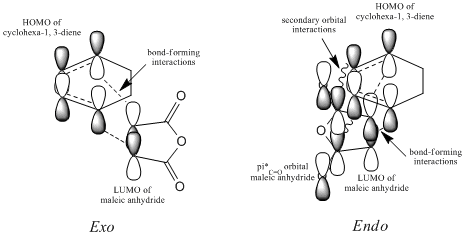
One can distungish between the geometries of the structures because in the exo-orientation, the substituents on the maleic anhydride, are pointing "up" away from the diene and the hydrogens are pointing "down". In theItalic text endo-orientation the substituents are pointing "down" towards the diene and the hydrogens are sticking "up".
Calculations show that the endo-transition structure exhibits a lower energy i.e it is more stable by 0.68kcalmol-1 than the exo-counterpart, which means the its activation energy is lower and thus confirms that it forms the kinetically controlled product, whilst the exo-transition structure corresponds to the product formed under thermodynamic control.
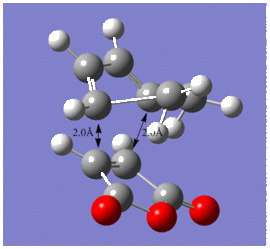
The various C-C bond lengths of the exo- and endo-transition structures were also compared as shown below;
 |
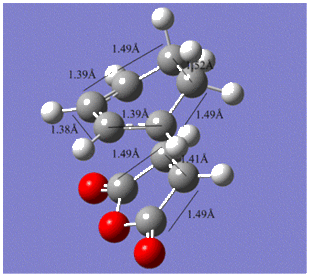 |
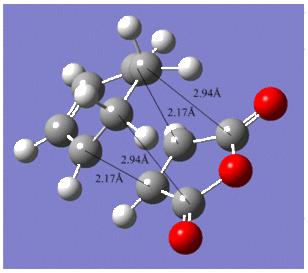 |
 |
The C-C bond lengths of both transition structures are very similar, including the lengths of the σC-C bond formations, 2.17Å in the exo- and 2.16Å in the endo-structures.
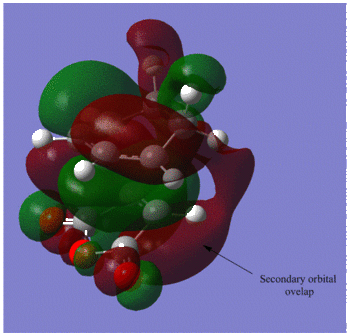
The C-C through space distances between the -(C=O)-O-(C=O)- fragment of the maleic anhydride and the C atoms of the “opposite” -CH2-CH2- for the exo is 2.94Å and the “opposite” -CH=CH- for the endo is 2.89Å. The shorter distance in the endo supports the fact that secondary orbital interactions can occur, whereas this stereoelectronic effect is absent in the exo.
The exo-form could be more strained due to the steric repulsion experienced by the -CH2-CH2- fragment and the maleic anhydride ring. In the endo-form, the steric interactions are between the -CH=CH- fragment and the maleic anhydride ring, which is less due to the sp2 rather than sp3 hybvridsation of the carbon atoms.
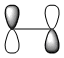
The HOMO and LUMO of both transition structures are tabulated below with their respective energies and symmetries;
| Molecular Orbital | Molecular Orbital Image | Energy/Hartrees (AM1) | Energy/kcalmol-1 | Symmetry w.r.t the plane | |
|---|---|---|---|---|---|
| Exo TS | HOMO | -0.34273 | -215.06616 | Anti-symmetric | |
| LUMO | -0.04045 | -25.38274 | Anti-symmetric | ||
| Endo TS | HOMO | -0.34505 | -216.52198 | Anti-symmetric | |
| LUMO | -0.03571 | -22.40835 | Anti-symmetric |
Both the HOMOs and LUMOs of each transition structure are anti-symmetric with respect to the plane of symmetry and it is the HOMO- LUMO overlap of the cyclohexa-1,3-diene and maleic anhydride respectively, which form the HOMO of the transition structures.
Both transition states exhibit primary HOMO-LUMO interactions leading to the formation of two σbonds. However, the preference for endo-stereochemistry is observed due to the overlap between the carbonyl group of the maleic anhydride and the developing pi bond at the back of the diene2. This interaction does not lead to the formation of new bonds but contributes to the stabilisation of endo-transition state with respect to that of the exo-one, suggesting that it is formed under kinetic control if the Diels-Alder reaction is irreversible. In contrast, the lack of this overlap in the exo-transition structure explains why this structure is higher in energy.
|
 |
References
- Bearpark. M. (2009). "The Transition State" Imperial College London. http://www.ch.ic.ac.uk/wiki/index.php/Mod:phys3
- Clayden. J, Greeves. N, Warren. S and Wothers.P. (2001) Organic Chemistry. Oxford University Press: 916
Conclusion
Computational stimulations to characterise transition structures on potential energy surfaces allows to successfully determine the preferred mechanisms of the reactions. Furthermore, by studying the molecular orbitals of the transition structures we can apply the Conservation of Orbital Symmetry to determine which reactions are allowed/forbidden as well showing the secondary orbital intercations which are very important in determining the regioselectivity of Diels-Alder reactions.