Rep:Mod2Proj:Yuko.Isayama3001
Mini Project; Phosphazene (Phosphonitrilic) Compounds
The project involved the investigation of the electronic structure of cyclic phosphazene trimers, (NPX2)3. Furthermore, the effects of substituents X (X=F, Cl, Br and NH2) on the vibrational properties of the phosphazene as well as the effects of increasing the trimer to specifically the cyclic tetramer will be studied.
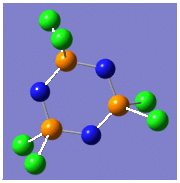
Structural and IR Analysis of (NPCl2)3
The geometric pararmeters as a result of the optimisation based on are given in the following tables with the experimental literature values:
| Bond length/± 0.01Å | ||
|---|---|---|
| Bond | Computed | Literature1 |
| P-N | 1.68 | 1.580 |
| P-Cl | 2.21 | 1.990 |
| Bond angle/± 0.1° | ||
|---|---|---|
| Bond | Computed | Literature1 |
| Cl-P-Cl | 100.8 | 101.4 |
| N-P-N | 117.0 | 118.4 |
| P-N-P | 123.0 | 121.4 |
Here is a summary table of the forms i.e. motion, frequency and intensity of each degenerate pair of the P-N-P vibration;
| Mode | Form of vibration | Computed frequency/±10cm-1 | Literature frequency2/±10cm-1 | Intensity |
| 1 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl_freq25.gif | 615 | - | 9 |
| 2 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl_freq26.gif] | 615 | - | 9 |
| 3 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl_freq29.gif | 1056 | 1218 | 1029 |
| 4 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl_freq30.gif | 1056 | 1218 | 1029 |
The computed P-N stretching frequencies based on the B3LYP method and LANL2DZ pseudo-potential and basis set of the other compounds of study;
| Characteristic P-N stretching frequencies/± 10cm-1 | ||
|---|---|---|
| Compound | Computed | Literature1 |
| (NPF2)3 | 1114 | 1297 |
| (NPCl2)3 | 1056 | 1218 |
| (NPBr2)3 | 1038 | 1157 |
(NPF2)3 DOI:10042/to-2754 (NPCl2)3 DOI:10042/to-2759 (NPBr2)3 DOI:10042/to-2752
Comparison of the computed bond lengths and vibrational frequencies of (PNCl2)3 with the experimental literature values show that there is a slight disagreement between them. Frequency analysis of other halocyclotriphosphazenes also showed discrepancies with the experimental literature values. Thus, it can be concluded that the LANL2DZ pseudo-potential and the associated basis set was not good enough to give results of high accuracy; in this method, the phosporous still only has a minimal basis set with the valence s and p orbital functions but it is known in theory that the low-lying d-orbitals of the phosphorous atom contributes to its hypervalency. Additionally, the change in the P-N-P vibration frequency in the halophosphazenes is partly due to the contraction of the 3d orbitals. Hence, another set of optimisation and frequency calculation were performed for each compound with the dAO functions added as an extra basis set which gave better agreement of results.
Structural and IR Analysis Based on the Basis Set with the dAO Functions
The geometric parameters as a result of the optimisation of (NPCl2)3 are given in the following tables:
| Bond length/± 0.01Å | ||
|---|---|---|
| Bond | Computed | Literature1 |
| P-N | 1.60 | 1.580 |
| P-Cl | 2.06 | 1.990 |
| Bond angle/± 0.1° | ||
|---|---|---|
| Bond angle | Computed | Literature1 |
| Cl-P-Cl | 102 | 101.4 |
| N-P-N | 114 | 118.4 |
| P-N-P | 126 | 121.4 |
Here is a summary table of the forms i.e. motion, frequency and intensity of each degenerate pair of the P-N-P vibration based on (NPCl2)3:
| Mode | Form of vibration | Computed frequency/±10cm-1 | Literature frequency2/±10cm-1 | Intensity |
| 1 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl2_freq26.gif | 847 | - | 16 |
| 2 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl2_freq27.gif | 847 | - | 16 |
| 3 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl2_freq29.gif | 1268 | 1218 | 1314 |
| 4 | https://www.ch.ic.ac.uk/wiki/index.php/Image:Phosp_Cl2_freq30.gif | 1268 | 1218 | 1314 |
Using the dAO basis functions as a basis set gives the same form of vibrations as the previous LANL2DZ basis set but in terms of the bond length and frequency, they are in better agreement with those of the experimental literature values than the previous method, suggesting that the d-orbitals have a significant contribution to both properties.
The geometric parameters as a result of the optimisation of the other compounds of study are given in the following table:
| Bond length/± 0.01Å | ||||||
|---|---|---|---|---|---|---|
| Bond | (NPF2)3 | (NPCl2)3 | (NPBr2)3 | (NPNH2)3 | (NPCl2)4 | |
| P-N | 1.58 | 1.59 | 1.60 | 1.62 | 1.57 | |
| P-X (X=F, Cl, Br, NH2) | 1.57 | 2.06 | 2.27 | 1.67 | 2.07 | |
(NPF2)3 DOI:10042/to-2763 (NPCl2)3 DOI:10042/to-2758 (NPBr2)3 DOI:10042/to-2767 (NPNH2)3 DOI:10042/to-2768 (NPCl2)4 DOI:10042/to-2770
The computed P-N stretching frequencies of the other compounds of study;
| Selected characteristic P-N-P frequency/± 10cm-1 | ||
|---|---|---|
| Compound | Computed | Literature2 |
| (NPF2)3 | 1337 | 1297 |
| (NPCl2)3 | 1268 | 1218 |
| (NPBr2)3 | 1240 | 1175 |
| (NPNH2)3 | 1250 | 1170 |
| (NPCl2)4 | 1457 | 1315 |
(NPF2)3 DOI:10042/to-2773 (NPCl2)3 DOI:10042/to-2757 (NPBr2)3 DOI:10042/to-2775 (NPNH2)3 DOI:10042/to-2777 (NPCl2)4 DOI:10042/to-2778
Frequency analysis of (NPCl2)3 and the other cyclotriphosphazenes show a strong characteristic absorption in the 1200/1300cm-1 region, which corresponds to P-N-P stretching mode, which is degenerate. The other degenerate modes of vibration at 847cm-1 are which was analysed in (NPCl2)3 represent the the forbidden symmetric P-N-P stretch.
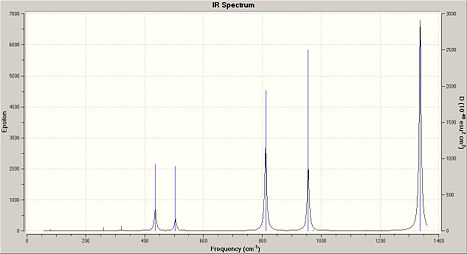 |
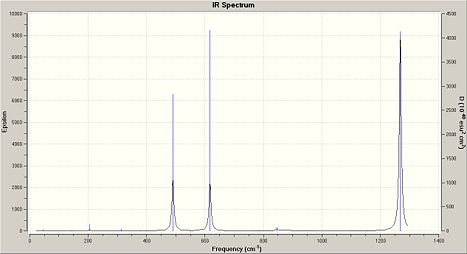 |
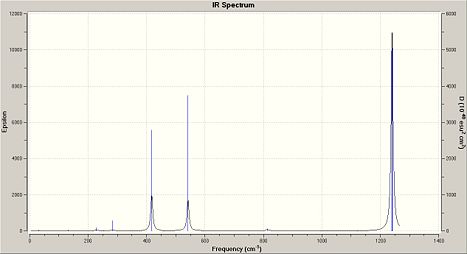 |
 |
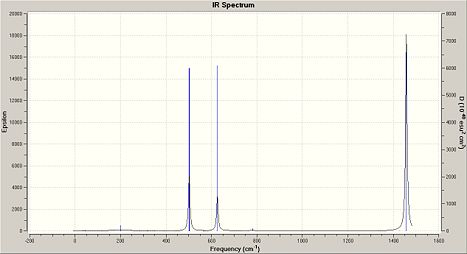 |
High electronegavtivity of the substituents increases the P-N stretching frequencies with the highest frequency found in (NHF2)3, whilst NH2 which is an electron-donating ligand, lowers it. This effect is attributed to the fact by withdrawing electrons from the phosphorous, the electronegative substituents increasingly permits nitrogen's ability to donate its lone pairs of electrons to the phosphorous. As a result, the P-N bonds appear stronger with higher vibrational frequencies. Another explanation is that the electron withdrawing effect of the ligands from the phosphrous may lead to the contraction of the 3d orbitals so that there is a more effective dpi-ppi bonding around the ring2. Either way, both explanations justify that the shortest P-N bond was found in (NHF2)3.
Geometric Analysis of the Phosphazenes
Optimisation of the all the cyclotriphosphazes present a planar cyclic arrangement, which could be furthed supported by calculating the dipole moment; a zero or near zero dipole moment would indicate near perfect planarity.
| (NPNH2)4 | |||
|---|---|---|---|
| Conformation | LANL2MB | LANL2DZ | Crystallised |
| Planar | Yes | ||
| Puckered | Yes | Yes | |
The puckered and planar conformations of the tetramer have an energy of -361.15585934 and -364.85859912AU, with an energy difference of 9721.46kJmol-1 which indicates that the planar conformation is more stable due to minimised non-bonding interaction between the chlorine substituents.
MO analysis of (NPCl2)3
Early interpretations of the bonding in phosphazenes were based on Dewar's island model3 which states that delocalization occurs via (P)dpi-(N)ppi overlap, resulting in a sigma-framework built from two sp2 hybrid orbitals of nitrogen and four sp3 hybrid orbitals of phosphorous, whilst the dxy and dyz orbitals of phosphorous form pi-bonds by interaction with the pz orbital of nitrogen. However, following the MO calculation of (NPCl2)3, the MO representations shown below suggest that the valence d-orbitals participate little to bonding; despite this, dAO functions are required in the calculations to give an accurate representation of the MOs, but they serve as polarization functions only4 (explained later).
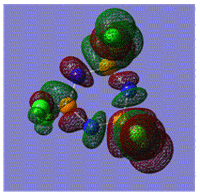 |
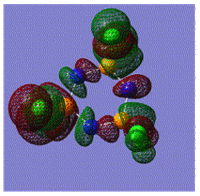 |
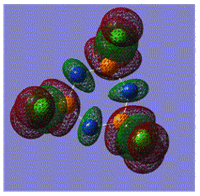 |
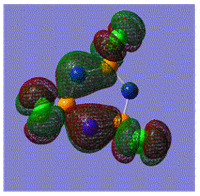 |
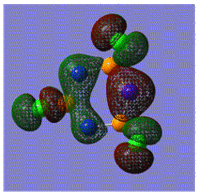 |
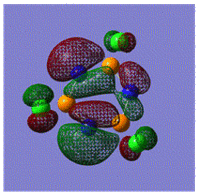 |
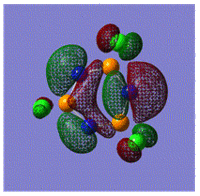 |
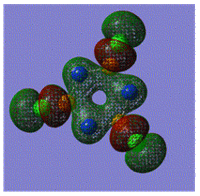 |
The MOs show that it is in fact pi'(in-plane) and pi(out-of-plane) bonding systems produced by the p-orbitals which are significant in planar cyclic triphosphazenes.
NBO Analysis of (NPCl2)3
Results show that a significant amount of electron density is dominated on the ring nitrogen, particularly on the 2p orbital where the natutral electron configuration is 4.94, which suggests that it is essentially an ionic system; the charge distribution of such system are best described in terms of a zwitterionic form5. This further supports the analysis of the MOs; electron density is not accumulated around the phosphorous atoms but rather the nitrogen atoms of the phosphazene. Having electron configurations of 1s2, 2s2, 2p3 and 1s2, 2s2, 2p6, 3s2, 3p3, for nitrogen and phosphorous atoms respectively, we would expect similar computed values for the natural electron configuration. However, the presence of the phosphorous 3d-orbitals lead to the polarisation of the nitrigen 2p orbitals, which explains the accumulation of electron densiy on the nitrogen 2p orbital.
Atom No Natural Electron Configuration
----------------------------------------------------------------------------
Cl 1 [core]3S( 1.90)3p( 5.31)
Cl 2 [core]3S( 1.90)3p( 5.31)
Cl 3 [core]3S( 1.90)3p( 5.31)
Cl 4 [core]3S( 1.90)3p( 5.31)
Cl 5 [core]3S( 1.90)3p( 5.31)
Cl 6 [core]3S( 1.90)3p( 5.31)
P 7 [core]3S( 0.90)3p( 2.03)3d( 0.12)4p( 0.03)
P 8 [core]3S( 0.90)3p( 2.03)3d( 0.12)4p( 0.03)
P 9 [core]3S( 0.90)3p( 2.03)3d( 0.12)4p( 0.03)
N 10 [core]2S( 1.54)2p( 4.94)3p( 0.01)
N 11 [core]2S( 1.54)2p( 4.94)3p( 0.01)
N 12 [core]2S( 1.54)2p( 4.94)3p( 0.01)
Atomic charges of the atoms also support this analysis, where the charge on nitrogen is -0.346 where the phosphorous has a charge of 0.403 as shown below:
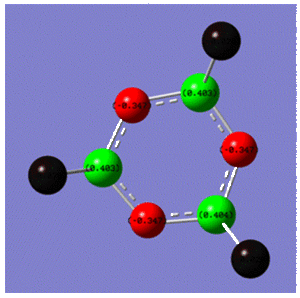 |
The pi and pi' bonding in phosphazene compounds are contributed by the p atomic orbitals, nevertheless computational results show that neglecting the dAO functions on the phosphrous atom shows discrepancies in the structure and geometry with experimental data, particularly the bond lengths and the frequency. It can be concluded that phosphazene frequency, consequently the bond length is not just affected by the electronegativity of the X ligands, but also ring size.
References
- G. I. Bullen, J. Chem. Soc. A 1450 (1971)DOI:10.1039/J19710001450
- H. R. Allcock, Chem. Rev. 72, 315 (1972) DOI:10.1021/cr60278a002
- M. J. S. Dewar, E. A. C. Lucken, M. A. J. Whitehead, Chem. Soc. 2423-2429 (1960)
- E. J. Magnusson, Am. Chem. Soc. 115, 1051-1061 (1993)
- M. Breza, Polyhedron, 19, 389 (2000)DOI:10.1016/S0277-5387(99)00368-X
- R. Jaeger, M. Debowski, I. Manners, G. J. Vancso, Inorg. Chem. 38, 1153 (1999)DOI:10.1021/ic980877n