Rep:Mod2:llama2822
Computational Labs Module 2: Inorganic Chemistry
Introduction
This wiki page reports a number of inorganic computational experiments done between the 8th and 16th of March. They comprise of three introductory molecules, that of borane, boron trichloride and bis(trichlorophosphine)molybdenum tetracarbonyl in both it's cis- and trans- form. These experiments will look into the uses of the program Gaussian with gaussview as a visual interface and will highlight the advantages and limitations of the program. Each experiment contains a separate discussion; regarding various observations from the results obtained and potentially highlights possible reasons and/or causes, a results section which documents the results obtained by tabulating the data and drawing graphs and finally an experimental section which complements the previous two sections by detailing the exact methods used to obtain the given results and describes the program in details.
The Modelling of Borane and it's Analogue
This first segment of the module regards the molecule borane and through modelling this will introduce the certain applications within Gaussian. This section will document the methods used to calculate the various models and inferences regarding the borane and boron trichloride molecules. The methods covered will have it's geometry optimised followed by vibrational and molecular orbital analysis.
Initial Modelling
Discussion
Table 1 shows the various data collected from calculations of the BH3 and BCl3 molecules. Both were run on two pseudo sets (being 3-21G and LanL2MB) to compare the resultant data. For the BH3 molecule the energies between the methods are very similar and the bond lengths do not greatly differ. This is because the LanL2MB method will treat the orbitals with quantum numbers 1 and 2 more or less the same compared to 3 and above, where the method deviates from the 3-21G set.
In comparison the B3LYP 3-21G method for the BCl3 molecule the method breaks down, and results in a high energy. This is due to the fact that the 3-21G pseudo potential does not distinguish between the electrons with quantum number 1,2 and the 3rd valence shell. Therefore it models these electrons with the same energy as the electrons already modelled, giving a large energy for the molecule.
Initially by looking at the bond lengths, the B-H distance is comparative to the literature value of 1.19Ǻ[1]. This was calculated through electron diffraction methods of diborane as borane is only present in the gaseous state, where it dimerises[2]. In comparison to the B-Cl bond length, which is longer due to the larger Cl atom, the literature value is 1.75Ǻ which is slightly lower than the calculated value of 1.78Ǻ.
Of interest is the Natural Bond Analysis where the occupancy of various orbitals is shown. It is clear that the borane structure has no occupancy in the pz orbital however in the boron trichloride structure the orbital is occupied. This is due to the donation of electrons from the chlorine p orbitals into the empty pz which stabilises the molecule. Therefore this shows reduced lewis acidity in comparison with the hydride variant as the unoccupied orbital is partially filled with electrons from the chlorine.
From looking at the second order pertubations of the BCl3 molecule, it was found that two of the E(2) energyies were over 20kcal/mol, which is that of number 23 and 26. These both have values of 40.97kcal/mol and have similar values for repeats. This is the interaction between one of the chlorine atoms (labelled 3) with the empty p orbital, supporting the conjugation of the molecule.
Results
| Molecule | Pseudo Potential Used | Total Energy/a.u. | Gradient/10-5a.u. | Bond Angle/o | Bond Length/ Angstroms | px Occupancy | pz Occupancy | |
| BH3 | 3-21G | -26.46 | 20.7 | 120 | 1.19 | / | / | |
| BH3 | LanL2MB | -26.29 | 0 | 120 | 1.17 | 0.79072 | 0 | |
| BCl3 | 3-21G | -1398.8 | 2.1 | 120 | 1.78 | / | / | |
| BCl3 | LanL2MB | -26.29 | 5.9 | 120 | 1.87 | 0.51473 | 0.36576 |
Table 1; Showing variance of bond lengths and occupancy with pseudo potential
| Molecule | Calculated B-H Bond Length/Angstroms | Literature B-H Bond Length/Angstroms | Calculated B-Cl Bond Length/ Angstroms | Literature B-Cl Bond Length/Angstroms | Bond Angle/o |
|---|---|---|---|---|---|
| BH3 | 1.19 | 1.19 | / | / | 120 |
| BCl3 | / | / | 1.78 | 1.75 | 120 |
Table 2; Bond lengths of the two modelled molecules
Molecular Orbital Modelling
Discussion
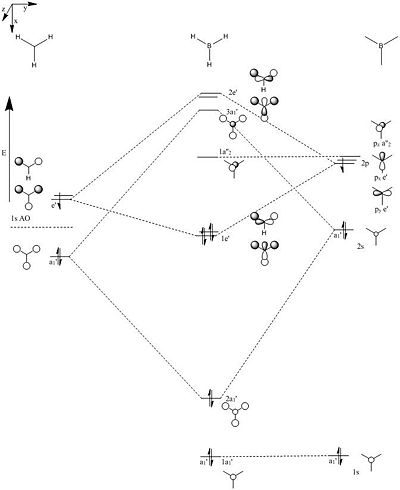
As shown in table 3 the calculated molecular orbitals are in line with the pictorial ones shown in the molecular orbital diagram, which correlate well. Of note is the comparison of the splitting of the degenerate e' and a1' orbitals, where the calculations show that the higher symmetry one is larger in energy. This is potentially due to the better s-s overlap in comparison to the s-p overlap shown in the e' orbitals.
From the calculated orbitals it is shown that there is a difference between the occupied and unoccupied orbitals, which is that the unoccupied ones are larger in size, or more diffuse. This could be explained by if they were occupied the nuclear shielding would be smaller for these orbitals therefore the attraction to the nucleus would be less for an electron in that orbital. Therefore it is less likely to be held tightly near the nucleus giving rise to a greater probability of finding the electron further away from the nucleus, giving rise to a larger molecular orbital surface.
Comparison of calculated and LCAO orbitals
The LCAO method gives an accurate description of the various molecular orbitals shown. Points such as where nodes are placed within the various orbitals and the occupying space of the orbitals are similar with both sets of orbitals, however the main difference is how desconstructive interference affects the lobes, such as molecular orbital 6, where the lobes of the p orbital are warped so that they point away from the interacting s orbitals from the hydrogen. I can also be said for molecular orbital 7 where one lobe of the p orbital is effectively gone. This lobe adds with the two s orbitals 120o behind it, which would not necessarily be obivious from the LCAO diagrams. Additionally it may not be clear that the 5th molecular orbital, that of the unoccupied pz, may not be widened over the whole molecule and not just localised on the boron.
Results
| Cartoon of Molecular Orbital | MO Number | Calculated Molecular Orbital Surface | Cartoon of Molecular Orbital | MO Number | Calculated Molecular Orbital Surface |

|
1 | 
|

|
2 | 
|

|
3 | 
|
|
4 | 
|

|
5 | 
|
|
6 | 
|

|
7 | 
|

|
8 | 
|
Table 3; The calculated and cartoon molecular orbitals of borane
Vibrational Modes
Discussion
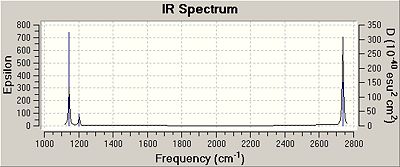
The initial vibration shown is of A``2 symmetry and is shown on the spectrum at 1144cm-1. The following two vibrations are degenerate and are labelled E' with a small peak showing on the spectrum. This is because there is only a small dipole change as the hydrogen atoms move slightly in space in the same plane as the boron atom, whereas in the first vibration they move through the horizontal plane, causing a larger dipole moment. Vibrational mode 4 is not shown on the spectrum and the reason is due to it's symmetry label being A'1, or in other words, totally symmetric which means it lacks a dipole change, meaning it is not observable in the spectrum. Again the last two vibrations are dengenerate and are labelled E'.
Results
Table 4; Data of the various vibrations of Borane
BCl3 Calculations
Discussion
The BCl3 molecule was drawn in Gaussian and the point group set to D3h with the tolerance set to very tight (0.0001). From this a gaussian input file was set up for geometry optimisation with a B3LYP-LanL2MB pseudo potential. Previously for the borane molecule 3-21G was used, however due to the more complex second row element chlorine the LanL2MB set was implemented. Also in the additional notes, pop=full was added and the NBO was changed to the full option. The method was then changed to the energy optimisation, which must use the same basis set as the geometry optimisation. If this is changed the occupation of the molecular orbitals will change and will not mirror the true result.
It might be that Gaussview opens a return file from gaussian that does not show any bonds, however this does not mean the atoms are bonded, but simply that the distance between the atoms are over the length prescribed by gaussview to draw a bond. The calculation still results in a bonding interaction between the atoms which is a distance which is longer than that determined by gaussview to draw a bond.
Chemical Bonding
A Chemical Bond could be defined as an electrostatic interaction between two atoms such as covalent, ionic, or hydrogen bonding. However electrostatic interactions such as dispersion forces aren’t considered as bonds so there must be a distinguishing feature between the two. First off chemical bonds can be considered permanent when compared with dispersion forces, meaning that there must be a strong favouring in energy to keep it bonded. Dispersion forces occur when electrons are imbalanced in a atom or molecule and a slight dipole is created, which creates another dipole in an adjacent group. Compare this with bonding, where the electrons are either in combined orbitals, or there is an excess of electron density on one atom in the bond with it being attracted to the nuclear charge on the other (ionic bonding) then it shows a clear difference, that electron are held or adapted in a position slightly different to that of its atomic state. It must be said that chemical bonding is only a minor perturbation in the atom orbital energies, however is significant in this case.
Experimental
The log files have been uploaded onto this server for the BH3[1] and BCl3[2] molecules.
Geometry Optimisation
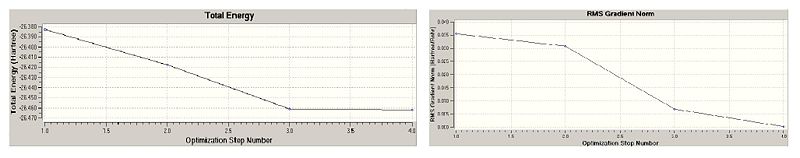
The borane molecule was created using the planar boron template which automatically selected hydrogens. With this the B-H bond length was changed from 1.18Ǻ that gaussview had originally to 1.5Ǻ, done by moving the hydrogen with respect to the boron. This model was then optimised using the DFT, B3LYP method with %chk=cab07_BH3_Geom_Opt as a filename. The resulting summary and output was saved and the log file looked at to produce the information. The optimisation resulted with a Energy of -26.46226338 a.u. and a gradient of 0.00020672 a.u. One of the output file was opened (checking the read intermediate geometries tab) and optimisation was selected to look at the optimisation diagrams and also the intermediates of this process.
Energy Optimisation
Once the geometry of the borane molecule had been optimised, the energy had to be for molecular orbitals to be produced. The Energy optimisation was selected on the calculations tab and the method set to DFT, B3LYP with a 3-21G Basis Set with pop=full written in the additional notes. Additionally on the NBO tab, full NBO was selected and the filename of %chk=cab07_BH3_Energy_Opt. This process took eight seconds to run and the checkpoint file was opened to look at the resultant orbitals, which were visualised by selecting the various ones and updating the program. The NBO analysis was taken from the output file.
Frequency Optimisation
Using the previous output file (unchecking the read intermediate geometries tab) and the Frequency optimisation selected on the calculations tab. The Method was DFT, B3LYP and the pseudo potential checked that it was the same as used last time, the 3-21G basis set. In the additional notes pop=(full,nbo) was written and a filename of %chk=cab07_BH3_Fq_Opt. The calculation took 8 seconds and the log file was opened from the calculation. From the results tab the vibration table was opened and the resultant vibrations were analysed.
Modelling of cis- and trans- bis(trichlorophosphine)molybdenum tetracarbonyl
''cis-'' bis(trichlorophosphine)molybdenum tetracarbonyl |
Jmol showing the cis isomer
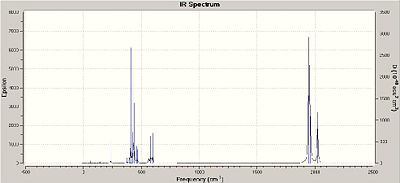
The two isomers which have been looked at, the cis- (DOI:10042/to-4563 ) and trans- (DOI:10042/to-4562 ) isomers, have different infrared spectra which vary on the number of carbonyl stretches observed. This is due to some of the stretches being completely symmetric and therefore not changing the dipole of the molecule, ultimately rendering the vibration IR inactive.
Discussion
Energy Analysis
From the geometry optimisations the trans- isomer is deemed slightly more stable (ca. 0.001 a.u. or 2.73kJ/mol), however with the accuracy of these calculations being approximately 10kJ/mol, this is a small difference. However the more stable trans- isomer could potentially be due to the minimalisation of interactions between the bulky phosphorous groups, with the high amounts of negative charge (ca. -0.1 on each Cl atom). Additionally the trans- isomer also minimises the dipole by 1 Debye.
It was also interesting to compare the various different conformations of the trans- isomer, where the phosphorous groups where arranged staggered, rather than eclipsed however this proved negligible (ca. 0.0001a.u. difference in favour of the staggered version) however this also resulted in a large decrease in dipole, to 0.0011 Debye compared to 0.3043 Debye.
A possible way of stabilising the cis- isomer with respect to the trans- one would be to put smaller groups on the phosphorous, possibly using methyl groups to result in a very stable trimethylphosphine ligand.
Vibration Mode Analysis
Through looking at the vibrations, mode 45 and 44 are the two that are absent in the trans- isomer spectrum. By looking at the cartoon for vibration 45, the symmetric stretch of all four CO ligands, it is clear that in the cis- isomer the molecule has a next dipole change pointing inbetween the axes of the CO ligands. In comparison the vibration for the trans- isomer is not present as the vibrations all cancel each other out in the CO ligand plane, resulting in a very low intensity which therefore means it is not present.
Vibration mode 44 is asymmetric stretch where the ligands trans- to each other are in phase with respect to the bond stretching, and not the direction of the stretch. Again this vibration is not seen in the trans- molecule as again the stretches cancel each other out, resulting in a very low intensity.
Vibration mode 43 shows the 2 CO ligands which are trans- to each other stretching in an asymmetric fashion, resulting in very similar frequencies and intensities for each isomer. Finally vibration mode 42 shows a cis- asymmetric stretch in the cis- isomer, and an almost identical vibration to mode 43 in the trans- isomer. This stretch is slightly lower in energy than # 43 which is due to a chlorine from each phosphine group being eclipsed to the carbonyl ligands in concern. A potential reason for this lower energy could be the chlorine atoms destabilising the CO bond, resulting in a weaker bond and therefore lower frequency of vibration.
The phosphine groups, which are pi electron acceptors much like the carbonyl ligands, with the d orbitals similar in size to the carbonyl antibonding pi molecular orbital. However the carbonyl ligand is still the stronger acceptor. This is observed in vibrational mode #42 as the trans- stretch is of higher wavenumber (higher Energy) than the cis- isomer. The cis- isomer has phosphine groups trans- to the stretching carbonyls and as the phosphine group is not as good an acceptor as the carbonyl ligand, more electron density goes onto the stretching carbonyl compared to the trans- isomer. Therefore more electron density in the antibonding orbital of the carbonyl means a weaker stretch and a lower frequency of vibration, as shown.
Additionally of note is that this vibrations (#42) the trans- isomer has a greater change in dipole as the stretching carbonyls are 180o to each other, compared to 90o in the cis- version. Therefore the trans- as a bigger dipole change as the vibrations are parallel to each other and add together, giving a much higher intensity, as shown in the results.
With respect to the IR spectra, none of the vibrations resulted in a negative frequency and those with low frequency vibrations (low wavenumber) result in low energy vibrations and at room temperature, the excited states of these vibrations are more populated than that of the high wavenumber frequencies. Therefore these vibrations are the ones that are most common.
Results
| Charge | Distribution | / Debye | ||||||
| Isomer | Conformation | Energy/a.u. | Dipole/Debye | Mo | P | Cl | C (trans- to PCl3) | C (cis-) |
| Cis- | Staggered | -623.58 | 1.31 | 0.19 | 0.32 | -0.12 | 0.07 | 0.04 |
| Trans- | Eclipsed | -623.58 | 0.3 | 0.08 | 0.3 | -0.12 | 0.08 | 0.1 |
| Trans- | Staggered | -623.58 | 0 | 0.01 | 0.3 | -0.12 | 0.09 | 0.09 |
Table 5; Energies of the cis- and trans- isomers
Table6; Showing the carbonyl vibrations of two isomers of Mo(PCl3)(CO)
Spectrum of isomers

Experimental
The cis- and trans- form was drawn into gaussview and the geometry optimised using tyhe B3LYP LanL2MB method and pseudo potential with a full nbo and opt=loose in the additional notes segment. Once converged, the geometries of the phosphorous groups of both compounds were changed slightly. In the cis- form one of the Cl's pointed along with the axial CO bond and a Cl on the other phosphorous group as aligned to the CO trans to the previous one, leaving a 'staggered' form. For the trans- isomer both of the phosphorous groups were eclipsed with one P-Cl bond aligned with a CO bond. With these new conformations the opt=loose is changed to read int=ultrafine scf=conver=9 and the pseudo potential changed to LANL2DZ.
From these geometeries the frequencies were determined by changing the method to frequency optimisation using the same method and same pseudo potential, the additional keywords were changed to int=ultrafine scf=conver=9 and once computed the vibrations were visualised.
Mini Project; The analysis of phosphazenes
Introduction
Phosphazenes have been a long standing component in inorganic chemistry and are currently of particular interest in polymer chemistry due to the strength in the P-N backbones. Phosphazene polymers potentially may have a wide range of uses, for example this paper documents a possible use for these polymers as grafts on organic molecules to make them water soluble[3], or as material with enhanced fire resistivity[4]. These examples depend on the P-N bond strength which is exhibited in the molecule hexachlorophosphazene, a molecule which mimicks benzene in aromaticity and first documented by Liebig[5]. The molecule itself has 4n+2 pi electrons ready to donate into the system, however additions to the atoms that make up the ring are relatively easy. This aromaticity was first thought to be due to p orbital interactions analogous to that in benzene. However further studies have proposed the idea that the d orbitals on the larger phosphorous atom contribute, and ongoing research via computational techniques have been inconclusive[6].
Aim
This project will first model the phosphazene complex by a number of methods and determine what method is the optimum one. Following on from this derivatives of the hexachlorophosphazene will be modelled swapping the chlorine atoms with electron donating methyl groups. These will be compared via their vibrational spectrum and their molecular orbitals. Finally the one of the phosphorous atoms will be replaced by arsenic and two methyl substituents will be added and difference isomers of this will be modelled and compared.
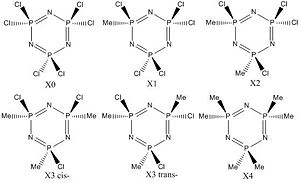
Methyl substituted hexachlorophosphazene
Discussion
From the initial calculation, it is clear that the B3LYP, 6-31G pseudo potential gave the best optimised geometry, with gaussview recognising the delocalisation of the electrons to form almost identical bond lengths around the ring. Additionally all the molecules drawn were planar, however the method for the LanL2MB broke down producing a distorted hexagon which did produce a low energy, however the optimisation was clearly not the best. Therefore the calculations carried on with using the 6-31G(d) psuedo potential.

cis- and trans- isomers
What is interesting to note is that there is no difference in energy between the cis and trans isomers with three methyl units around the ring. This hints that there is either little steric interaction between the groups, or that there is little energy required to distort the ring so that the methyl point away from each other. In fact by looking at figure 7, it shows that the trans version adopts a slight chair conformation, whereas the cis version distorts into a boat. This shows the relative ease at which the ring can be distorted and by distorting the molecule it starts to lose it's aromaticity. However despite the increased sterics the vibrational analysis shows the cis- isomer has both a higher wavenumber for ring breathing and planar to chair vibrations, meaning the ring is stronger in the cis- form.

Natural Bond Analysis
As previously discussed, debates continue about the contribution of the low lying 3d orbitals in the phosphorous to the aromatic system. It is interesting to compare the mono substituted derivative and the hexa substituted derivative and the difference that lies between the ring bonding. As shown in the table below, the hexamethylphosphazene has equivalent bonds throughout the ring with a contribution of 20% from the d orbitals in from the phosphorous. However for the phosphorous bearing the methyl group in the mono substituted ring, the d contribution is 41%, and for the rest of the phosphorous atoms it is 2%.
From looking at the orbital occupancies the phosphorous bearing the methyl group has a lower d occupancy (ca. 0.01), due to the methyl group not being a pi donor compared to the chlorine. In comparison the phosphorous bearing two chlorines have a higher d occupancy (ca. 0.03) and therefore are involved with backbonding with the chlorine and unable to interact with the aromatic system. Therefore these P-N bonds have a lower d orbital contribution, as shown in the table below.
Of additional note is the conformation of the mono substituted ring, which is puckered, compared to the completely planar hexa substituted ring. This is mirrored in the s orbital contribution, as this orbital is non-directional, and the directional (p orbitals) have slightly lower contributions in the bonding.
| methyl pentachlorophosphazene | hexamethylphosphazene | |||||||||||||
| Atom A | Atom B | Contribution | s/% | p/% | d/% | Atom A | Atom B | Contribution | s/% | p/% | d/% | |||
| P | N | 28% P, 72% N | P | 30 | 68 | 2 | P | N | 28% P, 72% N | P | 27 | 71 | 20 | |
| N | 34 | 66 | 0 | N | 36 | 64 | 0 | |||||||
| P | N | 8% P, 92% N | P | 0 | 59 | 41 | ||||||||
| N | 0 | 100 | 0 | |||||||||||
Table 7; Showing the natural bond order of the P-N bonds
Molecular Orbital Analysis
From the orbitals shown in Table 6 it is clear that as the methyl substituents are added to the ring, the electron density of the molecule moves into the ring. By using two extremes as a comparison, X0 and X4, it is shown that the HOMO moves from being centred on the chlorine atoms, to moving the majority of the density on the ring, and little on the methyls. It is also interesting to notes that the LUMO of X4 is mainly made up of the C-H antibonding and C-P bonding interactions, and two large lobes appear above and below the ring. These lobes come from the p orbitals that give the ring it's aromaticity. In comparison the LUMO of X0 is made up of the sigma antibonding interactions of both the P-Cl bonds and the P-N bonds and as the methyls are added, the vast majority of the LUMO remains on these P-Cl bonds. Also to note the 3 pointed lobe in the plane of the ring gradually disappears as you move along the series. This could potentially be due to the antibonding interaction of the P-N bonds being raised due to the less electronegative methyl groups being added and this interaction being raised too high in energy for it to be involved with the LUMO orbital.
Results
Table 8; Variation of methyl groups on substituted hexachlorophosphazene ring
| X0[7] | X1[8] | X2[9] | X3 cis[10] | X3 trans[11] | X4[12] | |||||||
| Frequency/cm-1 | Int/ε | Frequency/cm-1 | Int/ε | Frequency/cm-1 | Int/ε | Frequency/cm-1 | Int/ε | Frequency/cm-1 | Int/ε | Frequency/cm-1 | Int/ε | |
| Ring Breathing | 650 | 0 | 642.3 | 45.6 | 693 | 5.6 | 697 | 64 | 693 | 3 | 675 | 0 |
| Chair | 617 | 613 | 599 | 441 | 581 | 302 | 605 | 236 | 512 | 344 | 408 | 63 |
Table 9; The vibrations of molecules X0-X4
Mono substituted phosphazene ring derviative
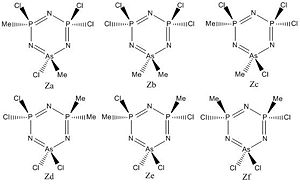
Discussion
The rings shown in figure six vary by only the placement of the two methyl groups around the ring. All six isomers were geometrically optimised and further to that the frequency optimisation was carried out, and five returned a result. Ths first observation to note is that gaussview does not recognise the delocalisation of bonds to the arsenic, however the P-N delocalised bonds still appear. Additionally,only Zb and Zd appear as fully planar forms, with the other isomers forming puckered rings. As shown on the graph in figure xx the frequencies vary only slightly for the majority of the vibrations, however for the ring transforming into the chair, a wide variety of frequencies were observed.

Aromaticity?
As previously gaussview does not recognise the delocalisation of the As-N bonds however still tries to adopt the planar ring form where possible (Isomers Zb & Zd) therefore there still must be an araomatic system present for this molecule to favour it, however the energy gain is low as higher steric systems (Zf, Zc) prefer the ring to be puckered, which lowers the aromatic strength. This is supported by the vibrations of the P-N-P bonds where the frequency is in the region of 1200-1210cm-1 for Zb & Zd, and of lower wavelength for Zf and Zc of around 1180-1190cm-1 (see table 11).
Results of mono substituted phosphazene ring
By looking at the table below, which compares the occupancy of the d orbitals in the fully methylated phosphazene ring and isomer Zb of the arsenic mono substituted series, the difference between the bonding is small. As discussed above the As-N bond is longer than the P-N bond by approximately 0.12 Angstroms however the orbital contributions largely remain similar. Using these similar environments (double methylated phosphorous/arsenic) it shows that the 4d orbitals on the arsenic are less populated than the 3d orbitals on the phosphorous, there is no change in orbital contribution. The lower occupancy could be due to the poorer overlap of orbitals of the As and N, due to the 4d orbitals being more diffuse above and below the plane of the ring and any interactions with the comparatively small p orbitals on the nitrogen being minimised.
However one inference of note is that of the contribution of the nitrogen. As discussed there is a poorer overlapy between the arsenic and the nitrogen, therefore there is a higher contribution from the p orbital of the nitrogen, as this will have greater overlap with the orbitals of the arsenic. This higher p character in the bond lowers the bond energy, and therefore gives rise to a longer bond length (1.76 Angstroms)and a weaker vibration (From ring breathing mode 640cm-1 [cf. 680cm-1 for non substituted ring]).
| P | As | As-N natural bond orbital analysis | ||||||||||
| 3 | dxy | 0.01497 | 3 | dxy | 1.99879 | Contribution/% | s/% | p/% | d/% | |||
| 3 | dxz | 0.02342 | 3 | dxz | 1.99979 | As | 29 | 24 | 75 | 1 | ||
| 3 | dyz | 0.01507 | 3 | dyz | 1.99929 | N | 71 | 23 | 76 | 1 | ||
| 3 | dx2y2 | 0.01619 | 3 | dx2y2 | 1.99946 | |||||||
| 3 | dz2 | 0.0085 | 3 | dz2 | 1.99894 | P | 28 | 27 | 71 | 2 | ||
| 4 | dxy | 0.00388 | N | 72 | 36 | 64 | 0 | |||||
| 4 | dxz | 0.00704 | ||||||||||
| 4 | dyz | 0.00783 | ||||||||||
| 4 | dx2y2 | 0.00832 | ||||||||||
| 4 | dz2 | 0.00374 |
Table 10; Showing natural bond orbital analysis of As-N bonds compared with P-N bonds
| Za[13] | Zb[14] | Zc[15] | |||||||||
| Stretch | Frequency | Infrared | Mode | Frequency | Infrared | Mode | Frequency | Infrared | Mode | ||
| /cm-1 | /10-40esu2cm2 | # | /cm-1 | /10-40esu2cm2 | # | /cm-1 | /10-40esu2cm2 | # | |||
| P(1)-N-P(4) stretch | 1187 | 793 | 36 | 1200 | 866 | 36 | 1196 | 801 | 36 | ||
| P-N-As stretch | 1123 | 890 | 35 | 1177 | 873 | 35 | 1123 | 879 | 35 | ||
| As-N stretch | 1038 | 113 | 34 | 1099 | 124 | 34 | 1034 | 109 | 34 | ||
| Ring Breathing | 675 | 23 | 26 | 675 | 9 | 27 | 676 | 31 | 27 | ||
| Ring transform to Chair | 573 | 249 | 23 | 349 | 0.7 | 20 | 591 | 143 | 24 | ||
| As-Cl stretch | 373 | 73 | 20 | \ | \ | \ | 386 | 90 | 20 | ||
| As-Me stretch | 628 | 25 | 25 | 625 | 47 | 26 | 627 | 11 | 25 | ||
| Zd[16] | Zf[17] | ||||||||||
| Stretch | Frequency | Infrared | Mode | Frequency | Infrared | Mode | |||||
| /cm-1 | /10-40esu2cm2 | # | /cm-1 | /10-40esu2cm2 | # | ||||||
| P-N-P stretch | 1228 | 740 | 36 | 1182 | 726 | 35 | |||||
| P-N stretch | 1103 | 961 | 35 | 1104 | 939 | 35 | |||||
| As-N stretch | 1042 | 204 | 34 | 1020 | 106 | 34 | |||||
| Ring Breathing | 645 | 10 | 25 | 664 | 30 | 25 | |||||
| Ring - Chair | 413 | 91 | 21 | 449 | 134 | 22 | |||||
| As-Cl stretch | 378 | 71 | 20 | 410 | 44 | 21 | |||||
| As-Me stretch | \ | \ | \ | \ | \ | \ |
Table 11; Showing vibrational frequencies of mono substituted arsenic phophazenes
Conclusion
This experiment has yielded two areas of results. Firstly how the addition of methyl group to the hexachlorophosphazene ring affects the respective molecular orbitals, ring bonding and vibrations of the molecule and secondly a comparison of isomers of these rings substituted with one arsenic atom. This report points out that there is a movement of electron density to the ring as more methyl substituents are added to the ring, and how the vibrations strengthen as more groups are added, showing greater bond strength with the removal of the chlorine atoms. Also it has shown that there is considerable d-orbital contribution to the bonding around the ring and therefore the aromatic system. In the second half of the experiment it has been shown the effects of adding an arsenic atom to the ring and increasing the methyl groups around this ring. It has considered it's vibrations and shows how they vary through the isomers.
Experimental
The molecule hexachlorophosphazene was drawn in gaussview and two methods were initially used to optimise it's geometry. One method was discarded (that of DFT, B3LYP LanL2MB) and the DFT B3LYP 6-31G (d) method was used to optimise all other derivative of the phosphazene ring. This optimised structure was modified into the other molecules that were going to be look at for this experiment.
From this 5 derivatives were made with increasing number of methyl substituents around the ring. These were then geometrically optimised and following this both and Energy optimisation (using the same method and full nbo) and Frequency optimisation. These yielded the molecular orbitals and vibrational spectra repectively.
From the phosphazene ring one of the phosphorous atoms was replaced by an arsenic and the geometry optimised. From this optimised structure two methyl substituents were added and moved around the ring to creat five isomers, which where then geometrically optimised using the same method, and also put into a frequency optimisation to yield their vibrational spectrum.
References
- ↑ Hedberg, K.; Schomaker, V.; J. Am. Chem. Soc.;1951.; 73 (4).; 1482 DOI:10.1021/ja01148a022
- ↑ Bauer S.H.; Chem. Rev.; 1942.; 31.; 43 DOI:10.1021/cr60098a001
- ↑ Gleria, M.; J. Fluorine Chem.; 2004.; 125.; 329 DOI:10.1016/j.jfluchem.2003.07.015
- ↑ Quinn, E. J.; Dieck, R. L.; J. Fire Flammability.; 1976.; 7.; 5
- ↑ Liebig-Wöhler.; Ann. Chem.; 1834.; 11 (1834).; 146
- ↑ Breza, M.; Polyhedron.; 2000.; 19.; 389DOI:10.1016/S0277-5387(99)00368-X
- ↑ DOI:10042/to-4632
- ↑ DOI:10042/to-4633
- ↑ DOI:10042/to-4634
- ↑ DOI:10042/to-4636
- ↑ DOI:10042/to-4635 |
- ↑ DOI:10042/to-4637
- ↑ DOI:10042/to-4602
- ↑ DOI:10042/to-4603
- ↑ DOI:10042/to-4604
- ↑ DOI:10042/to-4605
- ↑ DOI:10042/to-4606