Rep:Mod2:jg1208
James Gammerman (jg1208): Inorganic Chemistry Computational Laboratory
Introduction
Computational chemistry, sometimes called theoretical chemistry, or molecular modeling, is a field that can be said to be both old and young. It is old in the sense that its foundation was laid with the development of quantum mechanics in the early part of the twentieth century. It is young, however, insofar as arguably no technology in human history has developed at the pace that digital computers have over the last 35 years or so. With the digital computer as their ‘instruments’, computational chemists have taken advantage of this progress to develop and apply new theoretical methodologies at a similarly astonishing pace [1]
This project explores the applications of these methodologies to the realm of inorganic chemistry, in particular a method known as density functional theory (DFT)
The report consists of investigations into the optimal geometry and vibrational analysis of BH3, TlBr3 and the cis/trans isomers of Mo(CO)4PCl2. This is followed by a mini-project, which investigates pi-backbonding in metal-carbonyl complexes.
NB. There are many figures throughout this report - for the sake of page size, some of these have been made too small for the detail they contain to be visible. To see a larger version, please click on the image.
Background Information
Unlike other methods such as molecular mechanics, density functional theory allows for determination of electron distribution in a molecule, so that behaviour such as nucleophilicity and electrophilicity can be investigated.
Similar to ab initio and semi-empirical methods, DFT makes calculations based on the Schrodinger equation. However, it differs from the other two because DFT doesn’t calculate a wavefunction, but rather it derives the electron distribution (electron density function) directly (a functional is a function of another function). It calculates this using basis sets.
A basis set is a set of mathematical functions, normally representing atomic orbitals (AOs) - initially these AOs were used in the Hartree-Fock method (an ab initio method) and were represented as Slater orbitals, which corresponded to a set of functions which decayed exponentially with distance from the nuclei. Later, Frank Boys realised that the Slater orbitals could themselves be approximated as a linear combination of Gaussian orbitals. These Gaussian basis functions are much easier to compute, because they reduce the 4-centre integrals found in Slater orbitals to finite sums of 2-centre integrals and then 1-centre integrals. These are around 5 orders of magnitude quicker to solve than Slater orbitals, so Gaussian basis functions are much cheaper to use [2]
There are a number of different types of basis set:
- Minimal Basis Sets
These contain the minimum number of basis functions (i.e. AOs) needed for each individual atom. E.g. For H: 1s; For C: 1s, 2s 2px etc.
An example of this is STO-3G, which represents three Gaussian functionals (known as primitives) per Slater Type Orbital. [3]
- Split Valence Basis Sets
These are are larger basis sets and can be used for more complex systems - it increases the number of basis functions per atom. Examples include 3-21G and 6-31G, which have two basis functions of different sizes for each valence orbital. e.g. For the first orbital of H: 1s and 1s'. 3-21G is the simplest basis set which gives reasonable results. In this project is was used to loosely optimise molecules to a first approximation until bigger basis sets could be used.
- Polarised basis sets
Split valence basis sets allow orbitals to change size, but not shape. Polarised basis sets add in orbitals with angular momentum beyond that needed to describe the ground state which removes this limitation. For example, polarised basis sets add d functions to carbon atoms, and even add p functions to hydrogen atoms! Two examples which will appear in this project are 6-31G** and 6-311G**.
6-31G* is a significant improvement on 3-21G due to the added polarisation of atoms, and is considered the best compromise of speed and accuracy. It has become one of the most popular basis sets [4]
6-311G* is a further improvement as it adds more flexibility to the basis set.
- LACVP basis sets
To understand these we must define the term pseudopotential. Pseudopotentials are a special function that extend the basis set by modeling the core electrons of an atom - these simplify the calculation and allow chemists to focus on the valence electrons which dominate in bonding interactions. The LACVP series of basis sets is a combination of the 6-31G basis set with the LANL2DZ pseudopotential. Specifically the atoms H-Ar in the periodic table can be described with the 6-31G (or 6-31G*, 6-311G** etc) basis set while the heavier atoms are modeled using the LANL2DZ basis set. This was first employed in this project during the optimisation of TlBr3
Choosing which basis set to use is a key issue when performing computational calculations in chemistry, and this will be discussed throughout the project.
Geometry Optmisation of BH3
The point of a geometry optimisation is to find the minimum point on the potential energy surface of a molecule, which allows for prediction of its equilibrium structure. At minima on the surface the first derivative of the total energy, called the gradient, is zero. This can be shown on a graph, allowing us to determine whether optimisation has indeed taken place.
A BH3 molecule (Fig 1) was drawn on GaussView and all bond lengths were set to 1.5 angstroms. The geometry of the molecule was then optimised using the 3-21G basis set. As discussed above, this basis set gives the most reasonable quick calculation, so it was performed using the Gaussian program rather than the SCAN supercomputer. This DFT optimisation calculation employs the Born-Oppenhemier approximation, which solves the Schrodinger equation for the electronic distribution (or density) by moving the nuclei to different positions and measuring the energy in each place.
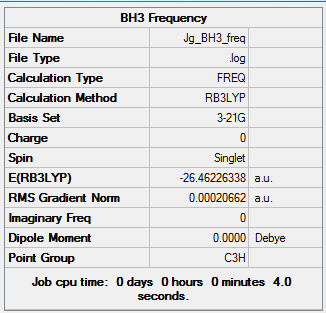
The log file for the optimisation is here:File:JG BH3 OPTIMISATION.LOG
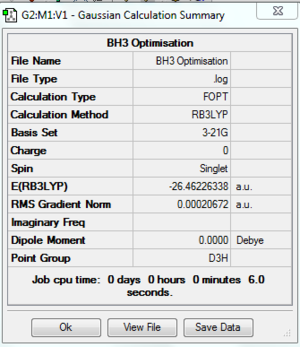
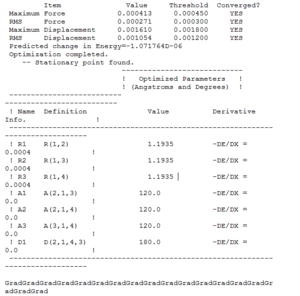
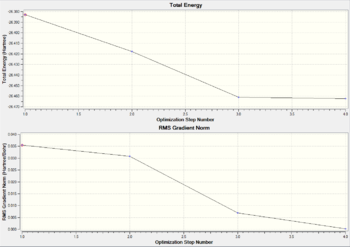
A summary of the optimisation is shown in Fig.2. To verify that the optimisation did indeed converge, the output text file from Gaussian is shown in Fig. 3 and the optimisation plots showing total energy and RMS gradient are shown in Fig. 4
 |
 |
 |
 |
| ||||
| Optimisation 1st Stage | Optimisation 2nd Stage | Optimisation 3rd Stage | Optimisation 4th Stage |
In the first two structures, the molecule appears to have no bonds. This is because GaussView only includes bonds that are shorter than some pre-determined length. Bearing in mind that the molecule is being optimised from a length of 1.5 angstroms to 1.19, it is understandable that GaussView includes bonds in the final two structures.
A jmol visualisation of the optimised BH3 molecule can be found
Finally, an animation of the optimisation was captured, and this is shown in Fig. 6

However, As mentioned above the second derivative of energy d2E/dR2 must be found to check that we have indeed found a minimum. This is done using vibrational analysis.
Vibrational Analysis
Energy calculations and geometry optimisations ignore the vibrations in molecular systems. In reality, the nuclei of molecules are constantly in motion. In equilibrium states, these vibrations are regular and predictable, and molecules can be identified by their chraceteristic spectra [3] A frequency calculation must use the same model and basis set that produced the optimised geometry - frequencies computed with different basis sets or procedures have no validity.
Here, DFT was used agin with the BLYP functional and 3-21G basis set to keep the calculations quick and reasonably accurate.
The IR spectrum produced along with its summary is shown in Fig.7. Analysis is given in Table 1.
The log file is here:File:JG BH3 FREQ.LOG
 |
 |
| Vibrational Form | Frequency | Intensity | Animation | Symmetry Label | |
|---|---|---|---|---|---|
| 1 | Wagging (out of plane) - The three hydrogens move down the z-axis, while the boron heads in the opposite direction | 1144 cm-1 | 92 |  |
A2 |
| 2 | Scissoring (in-plane) - the hydrogens move up and down in a concerted manner, increasing and decreasing the H-B-H angle | 1203 cm-1 | 12.3 |  |
E' |
| 3 | Rocking (in-plane) - two of the hydrogens oscillate in the plane of the molecule keeping a constant dihedral angle, while the other rocks from side to side | 1203 cm-1 | 12.3 |  |
E' |
| 4 | Symmetric Stretch - all the bonds expand and contract simultaneously, keeping the overall dipole moment constant | 2598 cm-1 | 0 |  |
A1' |
| 5 | Asymmetric Stretch - while one of the B-H bonds remains constant, the other two expand and contract in an alternating fashion | 2737 cm-1 | 103.7 |  |
E' |
| 6 | Asymmetric Stretch - two of the B-H bonds expand and contract simultaneously, while the third does the same but out of sync. | 2737 cm-1 | 103.7 |  |
E' |
As shown in the table, the signs of all the frequencies are positive which verifies that an energy minimum was found for the BH3 optimisation.
There are four atoms in BH3, so we would expect there to be (3x4 -6) six vibrational modes in the spectra. However, only three are seen. The reason for this is that we have to consider which of the vibrational modes are IR active, that is, which ones change the overall dipole moment of the molecule?
Inspection of the table reveals that stretch 4, a symmetric one, has no effect on dipole moment because its A'1 symmetry is retained throughout the stretching. Furthermore, modes 2 (scissoring) and 3 (rocking) occur at the same energy, so these two do not appear on the spectrum.
The three peaks found on the spectrum correspond are relatively a strong one at 1144cm-1 (wagging), a weak one at 1203cm-1 (rocking) and a strong one at 2737cm-1 (degenerate asymmetric stretch).
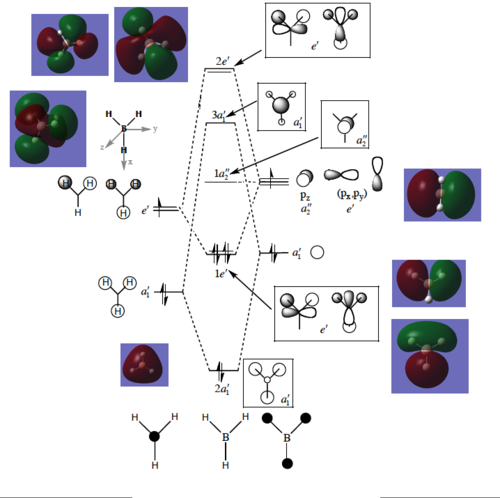
Molecular Orbital Diagram
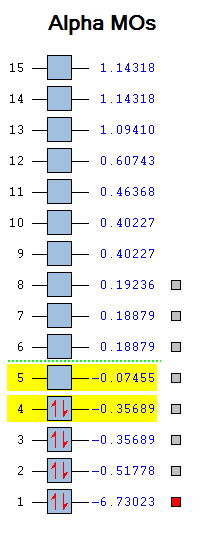
Having optimised the molecule using density functional theory, we now have a picture of the overall electron distribution and can use this to compute the molecular orbitals (MOs) for the molecule mathematically. We can compare these MOs to those found qualitatively by comparing them on an MO diagram, which follows in fig 8. Again, the B3LYP functional and 3-21G basis set was used, while the Gaussian calculation method was set to energy and an additional keyword "pop=full" used to compute the MOs.
 |
 |
|||
| ' | ' |
MO1 is not shown as it is too low in energy, while MOs 10 and higher do not participate in the orbital interactions very much, so are also omitted. The diagram shows an excellent match between the computational and LCAO methods - a testament to the reliability of qualitative MO theory as a way to describe bonding in compounds. The two methods most obviously overlap for the lower bonding orbitals - further up the diagram the resemblance is harder to see as the orbitals are more diffuse. The only significant difference between the two methods is the order of the top three MOs - the computational calculation finds the doubly degenerate 2e' orbitals higher than the 3a1' orbital as they are more destablised in this model.
Natural Bond Orbital Analysis
The concept of natural orbitals may be used for distributing electrons into atomic and molecular orbitals, and thereby deriving atomic charges and molecular bonds. The idea in natural bond orbital analysis is to use a one-electron density matrix to define the shape of the atomic orbitals in the molecular environment, and to derive molecular bonds from the electron density between atoms [5]
The output log file used to produce the MOs contains important information about the natural bond orbitals (NBOs). Gaussview can generate a charge distrbution picture of the molecule, which is displayed in Fig.9 to show the spread of electron density around BH3

|
||
| Quantified Charge Distrbution for BH3 |
The molecule's properties are further illuminated on inspection of the log file, displayed in Fig.10
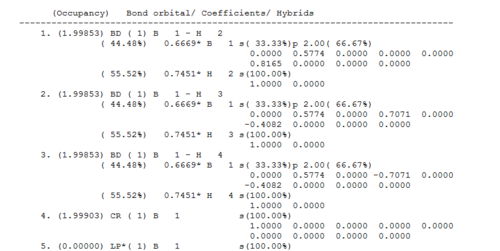
The log reveals the molecule hybridisation: the three contributions of boron in the B-H bonds have 2/3 p-character and 1/3 s-character i.e. they are sp2 hybridised. These hybrids interact with three hydrogen 1s atomic orbitals, a core 1s boron orbital and a lone pair, which we would expect to have 100% p-character. The 6-31G basis set calculated it to have 100% s-character, which is probably an error originating from the fact that 6-31G is quite a simple basis set.
Figure 11 shows the orbital energies and occupancies, and confirms that each bond consists of two electrons.

TlBr3 Analysis
Thallium is a bigger, more electron-rich molecule than the ones we have looked at thus far. This makes it harder to model using quantum mechanical methods like DFT because they show relativistic effects which make calculations harder to solve. The solution to this is to use a pseudopotential such as LANL2DZ (see background information). This basis set allows us to model heavy atoms like thallium by taking into account relativistic effects.
Geometry Optimisation
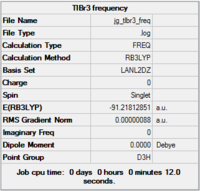
Initially the molecule was restricted to D3h symmetry, and then optimised using the B3LYP functional and LanL2DZ basis set. Once run, the output file was checked to make sure that the molecule had indeed converged. This is shown in Fig.12.

As shown, Gaussian managed to optimise the molecule. The optimised thallium-bromide bond distance is 2.65 angstroms, and the optimised bond angle is 120 degrees - the optimal angle for any planar molecule.
The log file for the optimised molecule can be found here: File:JG TLBR3 OPTIMISATION.LOG
The optimised TlBr3 molecule can be visualised as a jmol by clicking
The summary of the optimisation, along with the corresponding total energy and root mean squared gradent graphs are shown in Fig 13
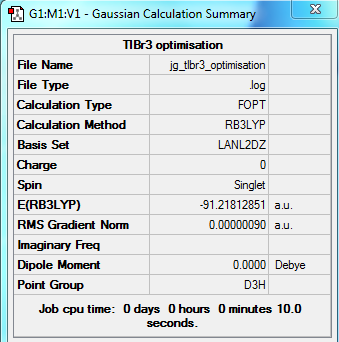 |
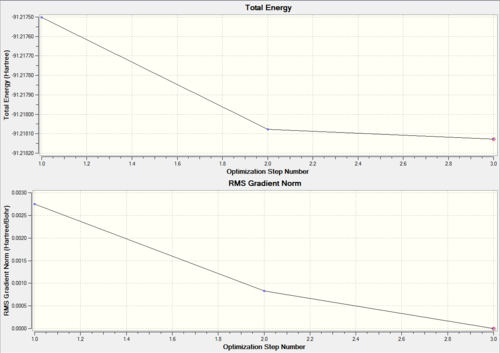 | |||
| Summary of Optimisation | Optimisation plots |
As before, the zero value of the RMS gradient confirms that the total energy has reached a turning point. The three steps in the optimisation are shown in Fig 14
 |
 |
 |
| |||
| Optimisation 1st Stage | Optimisation 2nd Stage | Optimisation 3rd Stage |
Frequency Analysis
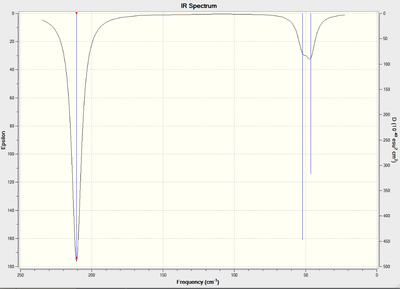
As for BH3, the molecule's vibrational modes were analysed using the same basis set and functional as in the optimisation. Here is the log file: File:JG TLBR3 FREQ.LOG Details of the vibrations can be found in the predicted spectrum and summary in Fig.15
 |
|
|||
| Summary of Frequencies | IR Spectrum |
These peaks are tabulated in table 2
| No | Vibrational Mode | Frequency [cm-1] | Intensity | Animation | Symmetry Label |
|---|---|---|---|---|---|
| 1 | Scissoring (in-plane) | 46.4 | 3.7 |  |
e' |
| 2 | Rocking (in-plane) | 46.4 | 3.7 |  |
e' |
| 3 | Wagging (out of plane) | 52 | 5.8 |  |
a2" |
| 4 | Symmetric Stretch | 165 | 0 |  |
a1' |
| 5 | Asymmetric Stretch | 210 | 25.5 |  |
e' |
| 6 | Asymmetric Stretching | 210 | 25.5 |  |
e' |

Like in BH3, some peaks vibrate at the same frequency (1 and 2, 5 and 6) so appear as one band on the spectrum. Peak 4 corresponds to a symmetric stretch so is not on the spectrum. As peaks 1 and 2 are so similar, they overlap to give the broad signal seen at 46cm-1.
The top line in Fig. 16 represents low frequencies arising from movement of the centre of mass of the molecule. These should be much lower than the lowest true vibrational frequencies, the first of which comes at 46.4cm - ten times higher. This value would be larger if a more accurate basis set were used such as the more recent Stuttgart-Dresden pseudopotentials developed by Dolg in 2002. That said, all the frequencies are positive which confirms that a minimum was reached during the optimisation.
What is a chemical bond?
To answer this question, who better to turn to than the two-time Nobel Prize winning chemist Linus Pauling? In 1939 he wrote his magnum opus The Nature of the Chemical Bond and the Structure of Molecules and Crystals in which he defined a chemical bond thus:
"We shall say that there is a chemical bond between two atoms or groups of atoms in case that the forces acting between them are such as to lead to the formation of an aggregate with sufficient stability to make it convenient for the chemist to consider it as an independent molecular species"
Such an interpretation is still definitely valid today, but a more common modern definition of the chemical bond is that it is the electrostatic interaction between opposite charges, which manifests itself as the sharing of electron density between nuclei in a covalent bond.
The strength of a chemical bond is related to its length, and since GaussView has a predetermined value for bond distance stored which it compares with calculated bond lengths, sometimes it doesn't show bonds that one might expect to be there. However, the presence or absence of these bond lines doesn't make a difference to calculations because a bond can be considered as a localised region of electron density between atoms, and using quantum mechanical methods such as DFT these regions are taken into account.
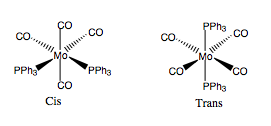
Cis and Trans Isomerism of MO(CO)4(PPh3)2
So we have now used DFT methods to optimise and characterise BH3 and TlBr3. We can now take this project further by investigating whether we can distinguish between the cis and trans isomers of the molybdenum complex MO(CO)4(PPH3)2 - their structures are shown in Fig.17. The way to do this is to predict the IR spectra of the isomers and compare, because group theory predicts that due to internal symmetry the cis isomer will four carbonyl bands, while the trans compound will only have one.
Since the large P(Ph3)ligands are large and take a long time to solve, the coordinated phenyl groups were replaced by chlorine atoms which are also relatively big and make a similar contribution to the bonding in the molecule.
Three basis sets were used to calculate the optimised geometries and vibrational frequencies - LanL2MB, LanL2DZ and a LanL2DZ set modified to include the d atomic orbitals of phosphorus, which shall herein be referred to as LanL2DZ(d)
Optimisation using LanL2MB
First of all, the isomers were drawn (Fig.17) and a loose optimisation was carried out using the B3LYP functional with the fairly weak pseudopotential LANL2MB - the convergence limit was set to loose in order to because here we are simply looking to get an idea of the approximate energies and bond lenths, not a serious calculation.
The summaries of these optimisations are shown in Fig.18
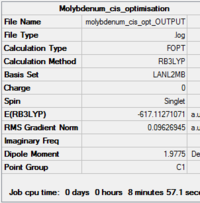 |
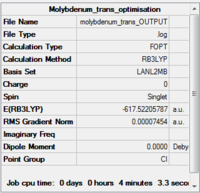 | |||
| Summary of loose optimisation of cis isomer DOI:10042/to-11970 | Summary of loose optimisation of trans isomer DOI:10042/to-11971 |
Visualisations of the optimised cis molecule can be found by clicking , and for the optimised trans molecule by clicking and animations of the optimisations can be found in Fig.
 |
 |
Optimisation using the LanL2DZ pseudopotential
The first optimisation at a low level supplies good bond lengths and angles, but does not describe dihedral angles so well, particularly the rotation of the PCl3 groups. This is because unless the molecule starts off in the right orientation, it often finds a local minimum rather than the global minimum. Therefore, for the cis conformer the torsion angle of the PCl3 groups was changed to 180 degrees, i.e. such that one Cl points up parallel to the axial bond, and one Cl of the other group points down, and for the trans conformer the PCl3 groups were made to be eclipsed, as shown in Fig...[6]

These altered structures were then optimised further with the B3LYP functional and LANL2DZ pseudopotential. LANL2DZ is a bigger pseudpotential than LanL2MB which can provide more accurate results, so the convergence was made tighter prior to the optimisation.
The summaries of the new optimisations are shown in Fig.22
 |
 | |||
| Summary of loose optimisation of cis isomer | Summary of loose optimisation of trans isomer |
From literature it is known that the equilibrium structure of the trans conformer is D4h, but the summary states that it is C1. This result is likely due to the fact that the geometry of the molecule was not restricted to D4h prior to optimisation - this can be achived via the point group tab in 'Edit' on GaussView.
The cis shows the same story - it should be C2v, but also turned out to be C1.
Animations of the optimisations are shown in Fig.23 & 24
 |
 |
Visualisations of the optimised cis molecule can be found by clicking , and for the optimised trans molecule by clicking
As mentioned above, to confirm that the geometry optimisation has truly found a minimum point, rather than a maximum, frequency analysis needs to be carried out. This is shown later in this part of the report.
Optimisation using d-orbital-adjusted basis set (optional)
Finally, a third optimisation was performed, in which the LanL2DZ basis set was modified manually using the Gaussian textfile to include the d-orbitals of phosphorus. This required addition of the keyword extrabasisinto the Gaussian input file.
The published files can be found here:
Cis isomer optimisation: DOI:10042/to-11988 Trans isomer optimisation: DOI:10042/to-11989
Geometry: Bond Angles & Lengths
| Cis Isomer | ||||
| Bond | Literature Length /Å | Loose Optimisation Length/ Å | LanL2DZ Optimisation Length /Å | dAO-modified LanL2DZ Optimisation Length/ Å |
| Mo-C axial | 1.97 | 2.06 | 2.06 | 2.02 |
| Mo-C equatorial | 2.02 | 2.10 | 2.01 | 2.05 |
| Mo-P | 2.58 | 2.53 | 2.51 | 2.47 |
| C-O axial | 1.13 | 1.19 | 1.17 | 1.18 |
| C-O equatorial | 1.15 | 1.19 | 1.17 | 1.18 |
| Trans Isomer | ||||
| Bond | Literature Length /Å | Loose Optimisation Length/ Å | LanL2DZ Optimisation Length /Å | dAO-modified LanL2DZ Optimisation Length/ Å |
| Mo-C | 1.85 | 2.11 | 2.06 | 2.06 |
| Mo-P | 2.50 | 2.48 | 2.45 | 2.42 |
| C-O | 1.16 | 1.19 | 1.17 | 1.17 |
The calculated values show an excellent agreement with the literature - for some of them such as the Mo-C (equatorial) and C-O bonds, they are almost exactly the same.
It is interesting to note that by and large the d-atomic orbital modified pseudopotential gave smaller bond lengths than the other methods - this is because the inclusion
of d-orbitals means that the bonds 'consist' of more electron density, so are shorter.
Vibration Analysis
Cis vibrational frequencies (initial): DOI:10042/to-11992
Cis vibrational frequencies (optional): DOI:10042/to-11990
Trans vibrational frequencies (initial): DOI:10042/to-11993
Trans vibrational frequencies (optional): DOI:10042/to-11991
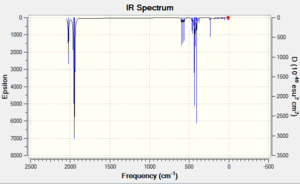 |
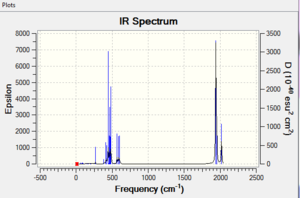 |
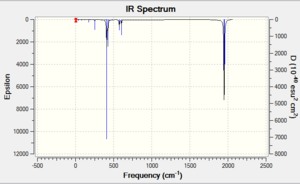 |
 |
Unfortunately there was insufficient time to produce a full table presenting the results with reference to literature [7] [8]. However, a comparison was made and they were found to be in good agreement. Furthermore, the d-atomic orbital modified basis set did generate slighty more accurate results, justifying their introduction.
Mini-Project: Investigating the effect of pi-backbonding on carbonyl ligands in a metal complex
Introduction
Since Ludwig Mond reported in 1888 that carbon monoxide (CO) reacts with crude nickel powder (the only metal to do so) to give a colourless, volatile compound C4NiO4, carbon monoxide has remained a curious and obscure ligand for chemists due to its versatility in coordinating to metal centres [9]
The carbon monoxide ligand, known as a carbonyl group, is particularly adept at stabilizing very low oxidation states: many carbonyl complexes such as Fe(CO)5 have the metal ion with an oxidation state of zero.
The bonding of CO to a metal centre can be described as follows. We picture the lone pair of the carbon atom as a Lewis sigma base (or electron-pair donor) and the empty antibonding orbital of the CO as a Lewis pi acid (or electron-pair acceptor), which receives pi-electron density from the filled d-orbitals on the metal atom. We can therefore picture the bonding in this molecule to consist of two contributions: a sigma bond from the ligand to metal atom and a pi bond from the metal atom to ligand. This type of pi-bonding is sometimes referred to as pi-backbonding. This is shown in Fig... [10]
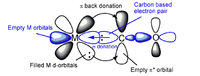
Carbon monoxide is not very nucleophilic, suggesting that sigma bonding to the d-metal atom is weak. Since many d-metal carbonyl complexes are very stable, the pi-backbonding contribution must be very strong, so we can conclude that the stability of these complexes comes predominantly from the pi-accepting ability of CO. This theory is corroborated by the fact that stable carbonyl complexes exist only for metals with filled d-orbitals with an energy suitable for donation to the CO antibonding orbital. The bonding of CO to a metal d atom can be described as being synergistic: the pi backbonding from the metal to the CO increases the electron density on the CO, which in turn increases the ability of the OC to form a sigma bond to the metal atom.
A more formal description of the bonding can be derived from the molecular orbital diagram of CO, which is discussed later in this mini-project.
What happens when the carbonyl group is bonded to different metal centres? The aim of this mini-project is to explore this question, with reference to three compounds: Free carbon monoxide (for comparison), V(CO)6 and Cr(CO)6.
The latter two have metals which are adjacent moving from left to right in the periodic table – one might therefore expect an increase in pi donation to the CO as the metals become more electron-rich. However, experiments have shown [12] that the opposite is in fact true: the dominant factor is the increase in nuclear charge and electronegativity values across the first period of the transition metals. This lowers the pi-donor ability of the metal centre, which in turn lowers the bond order of the M-C bond and thus increases ν(CO). Can DFT confirm this? If it can, which basis set is the 'best' (i.e. gives the value closest to experimental results)?
To investigate, the vibrational frequencies of these molecules were computed using various basis sets and compared with literature. The bond lengths in these molecules were also analysed to guage the reliablility of the basis sets in performing this investigation.
Basis set selection
As discussed in the background information, there are a many kinds of basis sets, and the more accurate ones often require more time (and money!) to be used.
For the metal complexes, the 6-31G basis set was chosen as it is a popular set which offers a good compromise in terms of speed and accuracy. The LanL2DZ is an effective core basis set which is better for heavier, inorganic atoms such as vanadium and chromium, so it is interesting to see how it compares to 6-31G.
For free CO, the 3-21G basis set was used to loosely optimise the molecule. It is the poorest of the basis sets, so was compared with 6-31G, which allows for polarisation of atoms, and 6-311G(d,p), which is a more flexible basis set.
Optimisation of Geometry
CrCO6
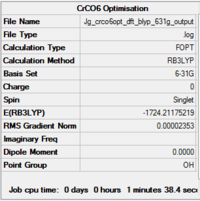
The molecule was first optimised using the 6-31G basis set, then using LanL2DZ.
The summaries of the optimisations are shown in Fig. 26... It should be noted that the molecule was restricted to Oh symmetry, which is the point group of monoloeptic hexacarbonyls [10]
 |
 |
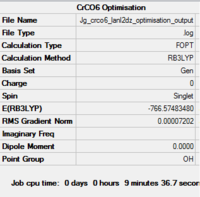 | ||||
| Summary of loose optimisation of CrCO6 using 6-31G DOI:10042/to-11974 | Animation of Optimisation of CrCO6 using LanL2DZ | Summary of tighter optimisation of CrCO6 using LanL2DZ DOI:10042/to-11980 |
The LanL2DZ optimised molecule can be visualised by clicking
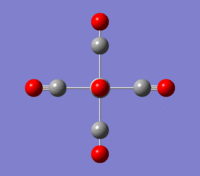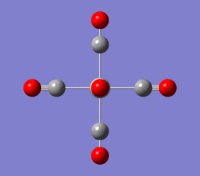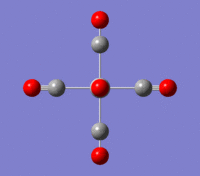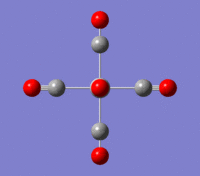
V(CO)6
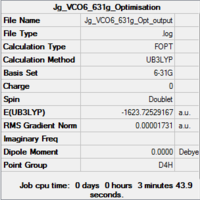 |
 |
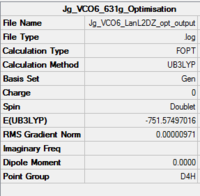 | ||||
| Summary of loose optimisation of VCO6 using 6-31G DOI:10042/to-11974 | Film of Optimisation of VCO6 using LanL2DZ | Summary of tighter optimisation of VCO6 using LanL2DZ DOI:10042/to-11980 |
The LanL2DZ optimised molecule can be visualised by clicking
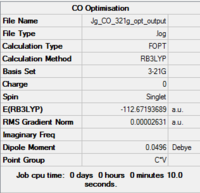
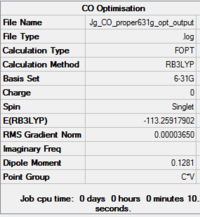
Free CO
 |
 |
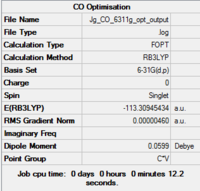 | ||||
| Fig...Summary of loose optimisation of free CO using 3-21G DOI:10042/to-11978 | Fig...Summary of optimisation of free CO using 6-31G DOI:10042/to-11982 | Fig...Summary of optimisation of free CO using 6-311G DOI:10042/to-11983 |
The LanL2DZ optimised molecule can be visualised by clicking
Bond Lengths
The bond lengths of the molecules were obtained from the output files of the two optimisations for comparison with literature. Pictures of the relevant sections of the output files are shown in Figs.... and the bond lengths are compiled and compared in Fig...
CrCO6
The bond lengths for the two sets are shown in Fig 27
 |

| |||
| Bond lengths/angles of CrCO6 using 6-31G | Bond lengths/angles of CrCO6 using LanL2DZ |
VCO6
The bond lengths computed for the two sets are shown in Fig.XXX
 |
 |
|||
| Bond lengths of VCO6 using 6-31G | Bond lengths of VCO6 using LanL2DZ |
Free CO
The bond lengths for the three basis sets used are shown in fig.XXX
 |
 |
 |
||||
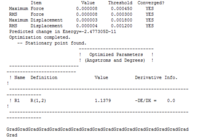
| Bond lengths/angles of Free CO using 3-21G | Bond lengths/angles of Free CO using 6-31G | Bond lengths/angles of Free CO using 6-311G |
Compilation & Discussion of All Bond Lengths (values closest to literature highlighted in blue)
 |
 |
| ||||
| Bond Length Analysis of CO | Bond Length Analysis of Cr(CO)6 | Bond Length Analysis of V(CO)6 |
The results provide an interesting insight into the accuracy of the basis sets.
For Free CO, as expected the 6-311G(d,p) basis set gave the best result, with a value within 0.02 angstroms of the literature. However, contrary to expectations the 6-31G set gave a worse value than the 3-21G set.
For the metal complexes the results differ: The LanL2DZ basis set is designed to be better suited to heavier metal atoms than 6-31G, and this is shown for the Cr(CO)6 molecule, in which LanL2DZ predicts all the bond lengths closer to the literature values. However, for V(CO)6 the LanL2DZ comes up short for the V-C bonds. We would not expect this, as it is the metal-carbon bonds which LanL2DZ should describe best! The answer to this problem can be found in the optimisation summaries: Unlike Cr(CO)6, when V(CO)6 was optimised the molecule was not subjected to restricted Oh geometry. Examination of the summary reveals that the optimised molecule has a point group of D4H. It is most likely that this error in the method of optimisation explains the unusually poor values for bond length obtained by the LanL2DZ basis set.
Further investigation would involve repeating this calculation, and also using other basis sets such as 6-311++G** which provides more diffuse functions, and 6-311++G(2df,2pd) which improves polarisation further.
Despite the problem incurred with V(CO)6, this has been accounted for, and the other evidence suggests that we have a clear idea of which basis sets are best for which purpose: For organic molecules like CO, the more flexible 6-311G basis set gives more accurate results than 6-31G and 3-21G, while for complexes with heavier atoms such as Cr(CO)6 and V(CO)6 the LANL2DZ basis set is more accurate than 6-31G. With this knowledge in mind, we can now move on to the vibrational analysis to investigate the pi-backbonding in these complexes.
Vibration Analysis
Most CO stretching bands occur in the range 2100-1700cm-1 and a prediction can be made about the number of active CO stretches in the IR and Raman spectra using group theory. If the CO ligands are not related by a centre of inversion or a threefold or higher symmetry axis, a molecule with N CO ligands will have N CO stretching absorption bands [10]. However, the hexacarbonyl complexes have a centre of inversion so we can predict that the computed spectra should have one main band in the region 2100-1700cm-1 - where it is exactly depends on the identity of the metal centre, and this will give us more information about the degree of pi-backbonding in the complexes.
Cr(CO)6
The output file of the optimisation was used to calculate the vibrational modes of the molecule using the SCAN supercomputer.
 |
 |
|
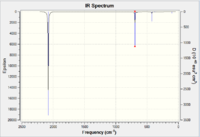 |
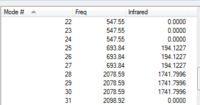
| ||||
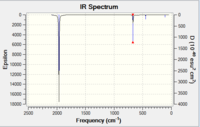
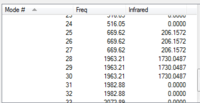
| IR Spectrum of CrCO6 using 6-31G DOI:10042/to-11975 | Table of main vibrational stretches using 6-31G | IR Spectrum of CrCO6 using LanL2DZ DOI:10042/to-11984 | Table of main vibrational stretches using LanL2DZ |
VCO6
The IR spectra were computed in the same way for V(CO)6
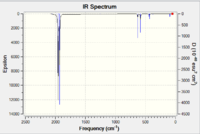 |
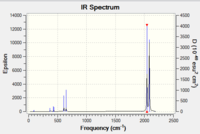 |
| ||
| IR Spectrum of V(CO)6 using 6-31G DOI:10042/to-11977 | IR Spectrum of V(CO)6 using LanL2DZ DOI:10042/to-11986 |
Free CO
The IR spectra were computed in the same way for free CO.
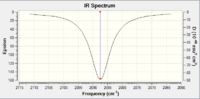 |
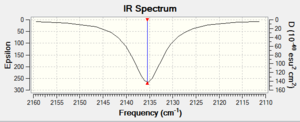 |
| ||
| IR Spectrum of Free CO using 3-21G DOI:10042/to-11977 | IR Spectrum of V(CO)6 using 6-311G(d,p) DOI:10042/to-11986 |
Discussion of Vibrational Analysis

Table XXX gives some rather surprising results. It shows that for both the metal complexes, the basis set which gave the value closest to literature was in fact 6-31G, not LanL2DZ, which is supposed to be better suited to heavier atoms. However, we must be wary of these results: Due to time constraints, the V(CO)6 molecule was not re-optimised to Oh geometry before vibrational analysis was undertaken - that is, the IR spectrum is likely to give slightly unreliable results. Moreover, time did not allow for repeats of this run.
.
The fact that 6-31G gave better results than LANL2DZ is definitely a surprise. The author of this report suggests that for further investigation, this experiment should be repeated, as it is known that SCAN does not always give completely accurate results, and runs should be repeated to improve reliability of data.
As predicted in the introduction, the computed spectra show that complexes with metals further to the right in the periodic table give higher v(CO)s. This is due to their higher electronegativity and nuclear charge, which reduces their tendency to donate their pi electrons to the metal in backbonding.There is therefore less electron density in the pi* antibonding orbitals of CO, so the CO bond itself is stronger, and thus vibrates a higher frequency.
Free CO, which obviously has no pi backbonding into the carbon pi* orbital, has a higher v(CO), and as expected it is found that the 6,311G(d,p) basis set gives a much more accurate value than 3-21G - within 8cm-1 of the literature value. Bearing in mind that the value for bond length was also very close to literature, we can summise that 6-311G(d,p) is an excellent basis set for characterising carbon monoxide.
Molecular Orbital Diagram of CO
The molecular orbitals of the CO molecule can be computed in the same way as for BH3 earlier in this report. Before construction of the diagram, we should note that 1) Oxygen is more electronegative than carbon, so its s and p orbitals are lower down and 2) the energy separation between 2s and 2p is greater in oxygen than in carbon. The MOs were calculated using the 6-311G(d,p) basis set in density functional theory by adding the key words "pop=full" into the Gaussian calculation. The final MO diagram showing combination of the AOs of C and O is given in Fig.XXX, along with a comparison of the computed MOs calculated in this experiment and in literature [13]
 |
 |
 |
| |||
| Molecular Orbital Diagram of CO, with Calculated Orbitals Superimposed | Experimental Computational Calculations of Molecular Orbitals | Literature Computational Calculations of Molecular Orbitals |
As we can see, the ground state configuration of CO is 1σ22σ21π43σ2
The 1σ orbital is mostly located on oxygen and is nonbonding [13]. The 2σ orbital is bonding. The 1π orbitals mostly consist of the doubly degenerate pair of bonding pi orbitals, with mainly C2p orbital character. The HOMO in CO is 3σ and is predominantly C2pz in character, largely nonbonding and located on the C atom. The LUMO is the doubly degenerate pair of antibonding pi orbitals, with mainly C2p orbital character.
This combination of frontier orbitals - a full sigma orbital largely localised on carbon and a pair of empty pi orbitals - is one reason why metal carbonyls are such a characteristic feature of the metals: in d-metal carbonyls, the HOMO lone pair orbital of CO participates in the formation of a σ bond and the LUMO antibonding pi orbital participates in the formation of pi bonds to the metal atom.
NBO Analysis
 |
 |
 |
| |||
| Charge Distribution of CO | Charge Distribution of V(CO)6 | Charge Distrbution of Cr(CO)6 |
Green (getting brighter) represents more positive charge, and red represents negative charge. The metal centres are red because they have a lot of electron density. We would expect that the chromium in the Cr(CO)6 complex would be lighter (i.e.have less electron density) than the vanadium complex, because going across the period there is a shielding effect. This is indeed what is noticed - V has a charge of -0.42, while Cr has a charge of -0.32.
In free CO, the oxygen is bright red, and the carbon is bright green, as expected: According to Sanderson electronegativity values, the difference in charge should be 0.900, and it is found in the analysis that the difference is (0.466x2) 0.932 - a very good agreement.
Conclusion
We have therefore completed our objective for this mini-project, to a certain degree: DFT has been used to explore the effect of pi-backbonding in metal carbonyl complexes. It has been shown that, in accordance with theory, the vibrational frequency of free CO is highest of all as there is no pi backbonding from a metal ion, and the vibrational frequency of the CO ligands in the complexes moves higher in wavenumber/frequency moving right across the third period as the metals become more electronegative and pi-backbond less to the CO. The results of the geometry optimisations have allowed for NBO analysis of the molecules, and MO analysis of carbon monoxide.
The accuracy of basis sets has also been explored, and the bond lengths gave an indication that the predicted variation in this accuracy is correct. However, vibrational analysis gave some rather strange results for the metal complexes, implying that LANL2DZ is in fact a less accurate basis set than 6-31G. Further investigation should be carried out to repeat this investigation and therefore test the reliability of the results found in this project.
References
- ↑ Cramer C.J.; Essentials of Computational Chemistry - Theories and Models; 2nd Ed.; John Wiley & Sons; p.1
- ↑ Young D.C. ‘Computational Chemistry: A practical guide for applying techniques to real-world problems’, John Wiley & Sons, Inc.; 2001, 336
- ↑ 3.0 3.1 Foresman J.B; Frisch E.; Exploring Chemistry with Electronic Structure Methods, 2nd Ed.; 1996, 97
- ↑ http://www.wavefun.com/support/sp_compfaq/Basis_Set_FAQ.html
- ↑ Jensen F.; Introduction to Computational Chemistry; 2nd Ed.; Wiley. p.309
- ↑ Hunt P.; 2nd Year Lecture Notes
- ↑ Ardon M.; J. Chem. Ed.; 2003, 80, 1249-1250
- ↑ Darensburg D.; J. Inorg. Chem.; 1979, 18
- ↑ Hill. A.F.; Organotransition Metal Chemistry, RSC Texts, 2002, 42
- ↑ 10.0 10.1 10.2 Atkins. P; Shriver & Atkins Inorganic Chemistry; 5th Ed.; 2010, 541
- ↑ Wilton-Ely J.; 3rd Year Lecture Course 'Organometallics', 2011
- ↑ Montgomery C.D.; J. Chem. Ed.; Jan 2007, 84, 1, p.102
- ↑ 13.0 13.1 Sharpe and Housecroft, Inorganic Chemistry, 3rd Ed.; Pearson Education, 2008 88