Rep:Mod1:shl108
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Objectives
The aim of this experiment is to use molecular mechanics to predict the regioselectivity and geometry of various molecules. In addition, semi-empirical and DFT molecular orbital theory is applied to investigate the regioselectivity and neighbouring group participation of bicyclic diene and the spectroscopic simulation in an organic molecule.
Modelling using Molecular Mechanics
Molecular mechanics applies the theory of Hooke’s Law to approximate the Morse curve which is a classical approach. This method determines the optimum geometry of a molecule by calculating a steric energy which is contributed by varies properties, such as stretching, bending, torsion, Van der Waals and hydrogen bonding energy. In this experiment, Allinger MM2 force field and MMFF94 field are employed by ChemBioDraw Ultra 12.0 to perform the geometry optimisation and the energy minimisation to identify the most stable conformation of molecules. Many force fields assign a point charge on a particular atom which is the point-charge electrostatics model. In contrast, the MM models assign dipoles to the bonds in a molecule which provide a more accurate calculation.
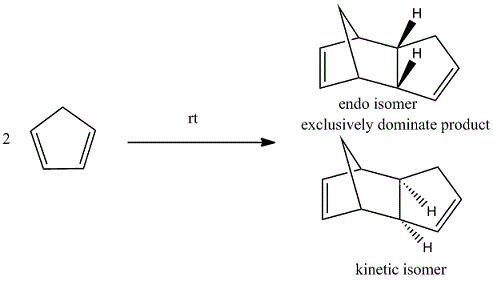
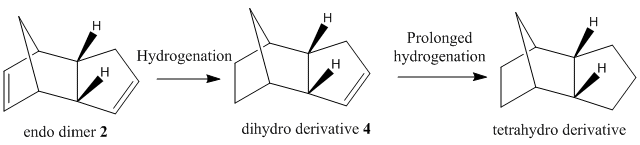
The Hydrogenation of Cyclopentadiene Dimer
| Dimer | Energy / kcal mol-1 | Energy / kJ mol-1 | |||
exo-dicyclopentadiene 1
|
31.88 | 133.46 | |||
endo-dicyclopentadiene 2
|
33.99 | 142.34 |
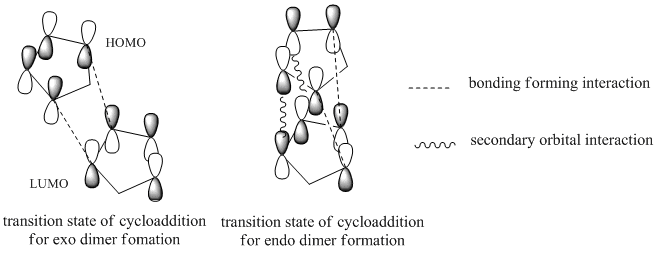
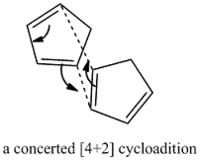
The cyclopentadiene dimers are synthesised by the dimerisation of cyclopentadiene. This reaction takes place spontaneously at room temperature by Diels-Alder reaction which is also known as [4+2] cycloaddition. As a result, the endo dimer is formed exclusively. Even at high temperature, only small amount of exo isomer is produced.[1] This can be explained by the Alder’s endo rule which is also known as the “principle of the maximum accumulation of unsaturated centers.” The regio and/or stereoselectivity of this dimerisation can be understood by Woodward-Hoffmann rules and secondary orbital interactions.
From table 1, the endo isomer has a higher energy, 142.34 kJ/mol, than the exo isomer, 133.46 kJ/mol. It means that the endo isomer is a kinetic product with a lower energy transition state which is formed at a faster rate, whereas exo isomer is a thermodynamic product vice versa. This also indicates that the reaction, in fact, is kinetically controlled and irreversible.
In conclusion, endo isomer dominates in this reaction.
| Contributions of Energy | Dihydro derivative 3 | Dihydro derivative 4 | |||||||
|
| ||||||||
| Energy/kcalmol-1 | Energy/kJmol-1 | Energy/kcalmol-1 | Energy/kJmol-1 | ||||||
| Stretching (Str) | 1.3 | 5.3 | 1.1 | 4.6 | |||||
| Bending (Bnd) | 19.9 | 83.1 | 14.5 | 60.8 | |||||
| Torsion (Tor) | 10.8 | 45.3 | 12.5 | 52.3 | |||||
| Van der Waals(VdW) | 5.6 | 23.6 | 4.5 | 18.9 | |||||
| Hydrogen Bonding (H-Bond) | 0.2 | 0.7 | 0.1 | 0.6 | |||||
| Total Energy | 35.7 | 149.4 | 31.2 | 130.4 |
In hydrogenation of endo dimer 2, D. Skála and J. Hanika[2] have reported that dihydro derivative 4 is formed exclusively before the prolonged hydrogenation. From the table 2 above, the MM2 force field has been used to optimise the confirmations for both dihydro derivatives 3 and 4. In addition, the total energy of dihydro derivative 4 is lower, 130.4 kJ/mol, which is due to the steric relif caused by the significantly reduced bending and Van de Waals energies. Therefore, dihydro derivative 4 is a thermodynamic product and this reaction is thermodynamically controlled.
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ Analogue) [3]
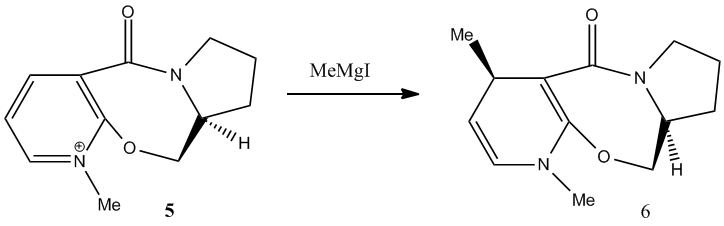
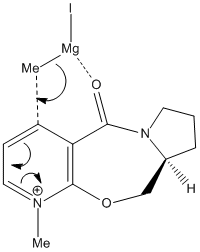
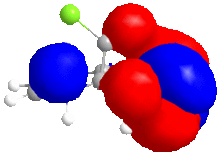
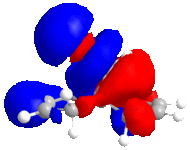
In this reaction, Grignard reagent [4], MeMgI, is used to react with optically active derivative of prolinol 5 by nucleophilic addition. In ChemBio3D, the element Magnesium is not recorded in the database, therefore, the software will not optimise the molecule when Grignard reagent is included. The mechanism postulated by Shultz et al [5] has explained the regio- and stereo- selectivities of this reaction. When the electropositive magnesium atom of the Grignard reagent coordinates the electronegative oxygen atom of the amide group, a stable six-membered ring transition state is formed. This coordination allows the methyl group to attack the top face of the adjacent C-4 position on the pyridinium ring in a concerted step.
From the data shown in table 2, the optimum conformation of prolinol derivative, conformer c, has a total energy of 179.2 kJmol-1. The energy is mostly contributed bend and Van der Waals energies. The optimal dihedral angle, (O)=C-C-C-H, is +10.4°.
| Contributions of Energy | Conformer a | Conformer b | Conformer c | |||
|
|
|
|
||||
| Energy/kcalmol-1 | Energy/kJmol-1 | Energy/kcalmol-1 | Energy/kJmol-1 | Energy/kcalmol-1 | Energy/kJmol-1 | |
| Stretching (Str) | 1.3 | 5.6 | 8.3 | 34.9 | 2.1 | 8.6 |
| Bending (Bnd) | 8.3 | 34.8 | 78.0 | 326.4 | 14.2 | 59.4 |
| Torsion (Tor) | 13.5 | 56.7 | 78.7 | 329.4 | 5.1 | 21.3 |
| Van der Waals(VdW) | 16.0 | 66.9 | 33.8 | 141.6 | 16.6 | 69.4 |
| dihedral angle | +23.4° | - | +66.5° | - | +10.4° | - |
| Total Energy | 44.1 | 184.6 | 193.5 | 810.2 | 42.8 | 179.2 |
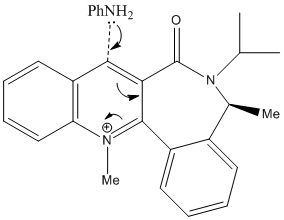
Pyridinium ring of 7 reacts with aniline to form 8 which is also by nucleophilic addition. However, the mechanism is slightly different to the reaction above,Grignard Reaction with Proline Derivative. It is due to a lone pair on the nitrogen atom of NH2Phenyl group repels with the lone pairs on the oxygen atom of the carbonyl group. Hence, the nucleophile will only attack opposite face to the carbonyl group.
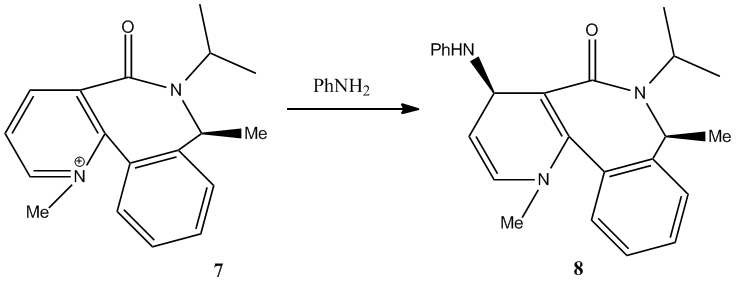
From the data obtained by MM2, conformer d is the most stable form of this molecule. It has a total energy of 265.6 kJmol-1 which is largely contirbuted by Van der Waals energy, 123 kJmol-1. In addition, the dihedral angle of O=C-C-C-H is -19.7° which is bottom face of the plane of naphthalene ring. Therefore, the nucleophile will attack the top face of the plane of naphthalene ring. It agrees with the experimental result.
| Contributions of Energy | Conformer d | Conformer e | Conformer f | |||
|
|
|
|
||||
| Energy/kcalmol-1 | Energy/kJmol-1 | Energy/kcalmol-1 | Energy/kJmol-1 | Energy/kcalmol-1 | Energy/kJmol-1 | |
| Stretching (Str) | 4.0 | 16.7 | 4.0 | 16.7 | 3.1 | 13.0 |
| Bending (Bnd) | 11.5 | 48.3 | 11.5 | 48.4 | 14.3 | 59.8 |
| Torsion (Tor) | 9.6 | 40.4 | 9.7 | 40.7 | 10.4 | 43.5 |
| Van der Waals(VdW) | 29.4 | 123.0 | 29.4 | 123.0 | 29.1 | 121.8 |
| dihedral angle | -19.7° | - | -20.8° | - | -24.8° | - |
| Total Energy | 63.4 | 265.6 | 63.6 | 266.4 | 66.4 | 278.0 |
In order to improve the calculation, MOPAC and Guassian Density Functional Theory (DFT), instead of MM2 force field, could be used to optimised the energy/conformation of the molecule. Both methods take a larger basis set in account which give more accurate results.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Atropisomerism arises when more than one conformer of a molecule cannot be distinguished because of the steric hindrance prevents the rotation about single bonds. In this reaction, the carbonyl group, C=O, cannot rotate across the ring due to the ring size and the electrostatics repulsion within this crowded electron density. Hence, the molecule has two isomers, with C=O bond pointing either upwards and downwards.
After the optimisation by MM2 force field, twist boat conformation is thermodynamically more stable than chair conformation. From the data in table 3, taxol intermediate 10 is thermodynamically more stable than taxol intermediate 9, 178.7 and 223.2 kJ mol-1 respectively. The major difference in energy distributions of these two isomers is the bending energy, 60.2 and 82.8 kJ mol-1 respectively. It means that C=O in taxol intermediate 9 experiences more repulsion within the steric ring and leads to instability. Moreover, for taxol intermediate 10, the carbonyl group and the hydrogen atoms adjacent to it stabilises the molecule further by interactions between the pi* C=O bond and the sigma C-H bond.
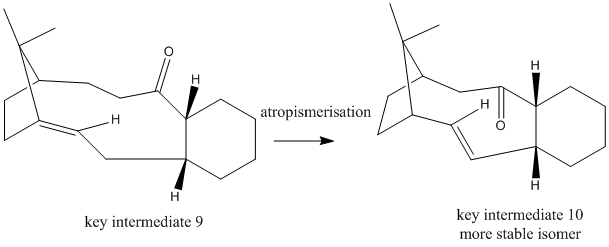
Furthermore, optimisation calculated by MMFF94 agrees the trend of the calculation processed by MM2. Because both methods use different basis sets to proceed the calculation. Hence, the values of the results cannot be compared. However, from the calculation by MMFF94, the taxol intermediate 10 is still the more thermodynamic product than taxol intermediate 9, 253.5 and 319.3-1 respectively.As a result, taxol intermediate 9 will isomerises spontaneously to taxol intermediate 10.
| Taxol intermediates | Stretching / kJ mol-1 | Bending / kJ mol-1 | Torsion / kJ mol-1 | Van der Waals / kJ mol-1 | Hydrogen Bonding / kJ mol-1| | Energy optimised
by MM2 force field / kcal mol-1 |
Energy optimised
by MM2 force field / kJ mol-1 |
Energy optimised
by MMFF94 force field / kcal mol-1 |
Energy optimised
by MMFF94 force field / kJ mol-1 | |||
Taxol intermediate 9
|
5.4 | 82.8 | 45.6 | 23.6 | 0.7 | 53.3 | 223.2 | 76.3 | 319.3 | |||
Taxol intermediate 10
|
4.7 | 60.2 | 52.4 | 18.8 | 0.6 | 42.7 | 178.7 | 60.5 | 253.5 |
Hyperstable olefin[6] is an explanation why some alkene reacts more slowly others. It is because of the extra stabilisation of the alkene. This kind of alkene is known as hyperstable olefin in which the alkene contains less strain than the parent alkane. In this case, the hyperstable olefin has a high activation energy which reduces its reactivity. The reactivity of the olefin can be compared with is olefin strain value. If the value is negative, the reactivity is low, vice versa. The rate of reaction can be speeded up by changing the reaction conditions, for instance, temperature and pressure, to affect the position of the equilibrium.
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
Reaction of Compound 12 with dichlorocarbene has been reported by Halton et al[7].
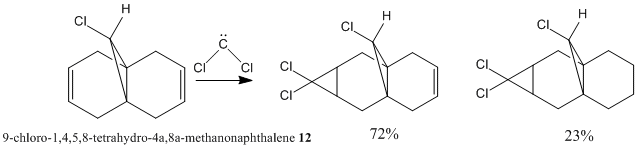
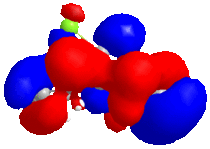
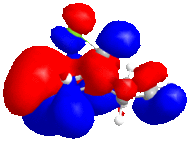
The five molecular orbitals of the compound 12 are simulated by MOPAC. By theory, HOMO and LUMO, which are the frontier orbitals, control the chemical reactivity of the reaction. The HOMO orbital of compound 12 shows that there is a higher electron density on the anti-alkene bond which makes it nucleophilic. Hence, the electrophile tends to attack this region which fits with the assumption. The stereochemistry of this reaction is also controlled by the orbitals. It can be noticed that the endo side of the antialkene has higher electron density which attracts the electrophile.
In addition, LUMO and LUMO+2 orbitals are the π* orbitals and the LUMO+1 is the the C-Cl σ* orbital. The LUMO+1 orbital interacts with the HOMO-1 orbital, the anti-alkene orbital, to show an antiperiplanar stabilisation. This causes the weakening of the C-Cl bond and can be identified by Infra-red spectroscopy. Moreover, the overall energy of the molecule is lowered. However, in the case of monoalkene, this interaction cannot be done due to the arrangement of the orbitals. These two orbitals do not interact as dialkene. Thus, the stretch of this C-Cl bond increases.
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
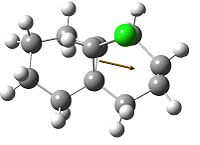 |
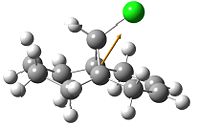 |
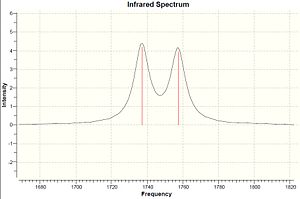
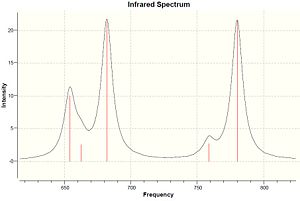
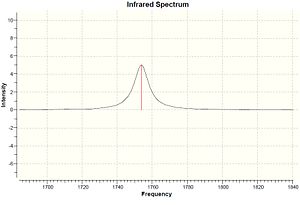
| Molecule | C-Cl stretch /cm-1 | syn C=C stretch /cm-1 | anti C=C stretch /cm-1 |
|---|---|---|---|
| Dialkene | 770.86 | 1757.34 | 1737.03 |
| Monoalkene | 780.03 | 1753.72 | - |
The IR frequencies shown on table 4 agree with the assumption explained above. The C-Cl stretch has a significant increase from dialkene to monoalkene, 770.86 to 780.03 cm-1, caused by the stabilisation of the interaction of LUMO+1 and HOMO-1 orbitals. The C=C stretches are expected as two for dialkene, 1737.03 and 1757.34 cm-1, and one for monoalkene, 1753.72 cm-1.
Structure based Mini project using DFT-based Molecular orbital methods
This project is about the electrophilic cyclization of methyl 3-oxo-2-(2-(prop-1-yn-1-yl)benzyl) butanoate to give the isomerism of indene derivative 13 and naphthalene 14. The DET method, mpw1pw91/6-311(d,p) is used to calculate the 13C NMR to differentiate the two isomers.
Electrophilic Cyclization of Acetylenic Malonates and Ketones
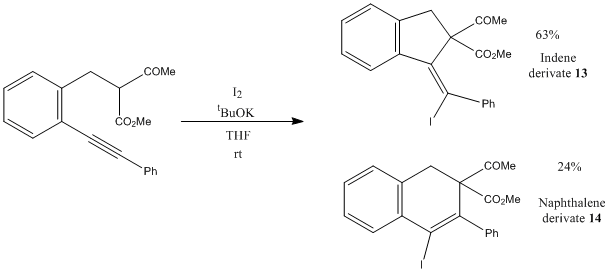
Liang and his co-workers[8] have performed the electrophilic cyclization of acetylenic malonates and ketones to form indene derivative 13 and naphthalene 14. In this reaction, the iodide can both carbon atoms of the alkyne bond in which the problem of regioselectivity arises. By attacking different position of nucleophilie carbons, five and six membered ring products are formed. From the energy data of both products and the mechanism postulated by the literature, this is a concerted reaction which is controlled kinetically.
| Contributions of Energy | 'Indene derivative 13' | ||||
|
|||||
| Energy/kcalmol-1 | Energy/kJmol-1 | ||||
| Stretching (Str) | 3.9 | 16.2 | |||
| Bending (Bnd) | 18.6 | 77.9 | |||
| Torsion (Tor) | -2.6 | -11.0 | |||
| Van der Waals(VdW) | 19.6 | 82.0 | |||
| Dipole/Dipole | 12.1 | 50.7 | |||
| Total Energy | 54.5 | 228.1 |
(R,Z)-Methyl 2-acetyl-1-(iodo(phenyl)methylene)-2,3-dihydro-1H-indene-2-carboxylate (13). Obtained as a solid. 13C NMR (75 MHz, CDCl3) δ 203.5, 171.1, 145.2, 144.6, 143.0, 139.7, 129.7, 129.1, 128.3, 127.8, 126.2, 126.0, 124.4, 92.0, 73.5, 52.4, 42.0, 28.0; IR (KBr, cm-1) 3424, 1733, 1710, 1248, 1079, 1028;
| Contributions of Energy | 'Naphthalene derivative 14' | ||||
|
|||||
| Energy/kcalmol-1 | Energy/kJmol-1 | ||||
| Stretching (Str) | 4.4 | 18.5 | |||
| Bending (Bnd) | 13.2 | 55.5 | |||
| Torsion (Tor) | -3.1 | -12.8 | |||
| Van der Waals(VdW) | 23.3 | 97.7 | |||
| Dipole/Dipole | 8.6 | 36.1 | |||
| Total Energy | 51.3 | 214.9 |
(S)-Methyl 2-acetyl-4-iodo-3-phenyl-1,2-dihydronaphthalene-2-carboxylate (14). Obtained as an oil. 13C NMR (75 MHz, CDCl3) δ 201.6, 170.6, 146.9, 145.7, 142.9, 138.4, 129.2, 128.7, 128.5, 127.6, 126.9, 125.2, 124.8, 97.7, 74.1, 52.9, 41.2, 28.7; IR (neat, cm-1) 3433, 1733, 1708, 1253, 1161, 1064;
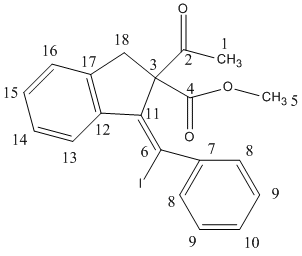
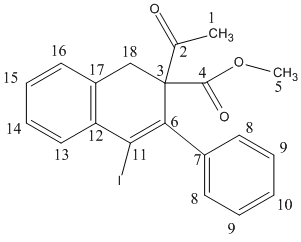
| Carbon | Indene derivative 13 | Carbon | Naphthalene derivative 14 |
| 2 | 203.6 | 2 | 202.1 |
| 4 | 171.5 | 4 | 170.3 |
| 12 | 145.9 | 12 | 146.5 |
| 17 | 144.8 | 17 | 145.8 |
| 7 | 144.1 | 7 | 143.2 |
| 16 | 139.5 | 16 | 141.9 |
| 13 | 130.1 | 13 | 138.9 |
| 14 | 129.7 | 14 | 129.6 |
| 9 | 128.0 | 9 | 129.2 |
| 15 | 126.7 | 15 | 128.9 |
| 10 | 126.0 | 10 | 127.9 |
| 8 | 125.6 | 8 | 125.3 |
| 11 | 124.5 | 6 | 124.9 |
| 3 | 91.4 | 3 | 98.8 |
| 18 | 74.1 | 18 | 74.3 |
| 6 | 52.1 | 11 | 51.4 |
| 1 | 41.9 | 1 | 41.0 |
| 5 | 28.0 | 5 | 29.1 |
By comparing the 13C NMR chemical shift obtained from experimental and DFT, there is only an error within 2 ppm observed. In addition, the 13C NMR of the experiment cannot be integrated due to the natural abundance of 13C is low. However, the calculated 13C NMR has shown pleasant integration of 13C in each environment. This shows the DFT can make a fairly accurate prediction to the small size molecule like this example.
The main difference in the 13C NMR of the two isomers is the chemical shift of C-6 and C-11 has changed significantly due to the different substituents of the alkene bond. Both iodide and phenyl group shift the chemical shift of the carbon of the alkene to the RHS of the spectrum. The C-6 of the indene derivative 13 has experienced the most of this effect. Moreover, in napthalene derivative 14, the chemical shift of C-6 increases because the bulky iodide group is no longer attached at this position.
References
- ↑ Pierluigi Caramella*, Paolo Quadrelli, and Lucio Toma, An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the Endo Dimerization of Cyclopentadiene, 2002, 124, 1130-1131: DOI:10.1021/ja016622h
- ↑ D. Skála, J. Hanika, Pet. Coal, 2003, 45, 105: PDF
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
- ↑ Y.H. Lai, Synthesis, 1981, 1981, 585: DOI:10.1055/s-1981-29537
- ↑ A.G. Shultz, L. Flood, J.P. Springer, J. Org. Chem., 1986, 51, 838: DOI:10.1021/jo00356a016
- ↑ Hyperstable Olefins: Further Calculational Explorations and Predictions, JACS 1986, 108, pp3951-3960: DOI:10.1021/ja00398a003 ]
- ↑ B. Halton, S.G.G. Russell, J. Org. Chem., 1991, 56, 5553: DOI:10.1021/jo00019a015
- ↑ H. P. Bi, L. N. Guo, X. H. Duan, F. R. Gou, S. H. Huang, X. Y. Liu, and Y. M. Liang, Org. Lett., 2007, 9 (3), pp 397–400: DOI:10.1021/ol062683e