Rep:Mod1:em207
Edward Moore Third Year computational labs Module 1
Introduction:
Computational chemistry is a very powerfull tool that has developed grealy over the last decade, being used by synthetic chemists to validate the accuracy of previous assumptions or to indead aid with predictions.
The Hydrogenation of Cylopentadiene Dimer
Endo vs Exo dimers
In this section the energy and structures of endo and exo dimers of cyclopentadiene were firstly investigated. The structres were drawn in ChemDraw 3D and the molecular dynamics program MM2 run. The resutls of this section are shown bellow.
| type of energy contribution | energies for endo kcal/mol | energies for exo kcal/mol |
|---|---|---|
| Strech | 1.259 | 1.281 |
| Bend | 20.854 | 20.591 |
| Strech-Bend | -0.836 | -0.838 |
| Torsion | 9.507 | 7.639 |
| Non-1,4 VDW | -1.525 | -1.406 |
| 1,4 VDW | 4.300 | 4.239 |
| Dipole/Dipole | 0.446 | 0.377 |
| Total Energy | 34.005 | 31.883 |
From the above table it can be seen that the endo product (which is the favoured) must be the kinetic product and not the thermodynamic as the energy of the exo dimer is lower than that of the endo dimer. The reason the kinetic product is less thermodynamicaly stable is that the tortional energy of the endo is greater than that of the exo dimer.
Hydrogination within 5 membered ring vs 6 membered ring
In this section the relative enegies the products of hydrogination across the five and six membered rings of the cyclopentadiene were calculated in ChemDraw 3D using MM2 the results are shown below.

| type of energy contribution | energies for hydrogination of 6 membered ring kcal/mol | energies for hydrogination of 5 membered ring kcal/mol |
|---|---|---|
| Strech | 1.131 | 1.103 |
| Bend | 13.015 | 14.515 |
| Strech-Bend | -0.562 | -0.546 |
| Torsion | 12.424 | 12.512 |
| Non-1,4 VDW | -1.333 | -1.407 |
| 1,4 VDW | 4.383 | 4.499 |
| Dipole/Dipole | 0.141 | 0.141 |
| Total Energy | 29.255 | 31.177 |
From the results it can be seen that the 6 membered ring hydrogination product is more thermodynamically stable compared with the 5 membered ring. This is largly due to the fact that the bending energy and the 1,4 Van Der Vaals are significantly lower in the 6 compared to the five membered products.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Methylation via a MeMgI
In this section the effect of altering the dihedral angle were measured and observed using the MM2 program.

| Type of energy contribution | Energies of NAD+ anolouge starting orientation out of the plane kcal/mol | Energies of NAD+ anolouge starting orientation in plane with oxygen on outerside of molecule kcal/mol | energies of NAD+ anolouge starting orientation into the plane kcal/mol | Energies of NAD+ anolouge starting orientation in plane with oxygen on inerside of molecule kcal/mol |
|---|---|---|---|---|
| Strech | 1.328 | 1.328 | 1.328 | 3.890 |
| Bend | 8.189 | 8.189 | 8.189 | 115.818 |
| Strech-Bend | 0.121 | 0.121 | 0.121 | -0.176 |
| Torsion | 13.685 | 13.685 | 13.685 | 33.218 |
| Non-1,4 VDW | -1.654 | -1.654 | -1.654 | 2.775 |
| 1,4 VDW | 16.024 | 16.024 | 16.024 | 25.454 |
| Charge/Dipole | 10.334 | 10.334 | 10.334 | -7.398 |
| Dipole/Dipole | -3.848 | -3.848 | -3.848 | -5.084 |
| Total Energy | 44.124 | 44.124 | 44.124 | -168.499 |
From the above table it can be seen that when the carbonyl is orentated at 180o to the starting position it can be seen that the energy of the moleuce is greatly increased this is due to the incerased torsional and bend energies. This is due to the increased sterics associated with having the carbonyl within the ring.
It may be sujested if the attack angle were investigated further that when the nucleophile were to approach allong the least hindered rout that the carbonyl would be at 108o.
Next the energies of the starting materials were compared to the products, using MM2.

| type of energy contribution | Energies of NAD+ anolouge kcal/mol | Energies of methylated NAD+ anolouge kcal/mol |
|---|---|---|
| Strech | 1.328 | 1.500 |
| Bend | 8.189 | 12.469 |
| Strech-Bend | 0.121 | 0.334 |
| Torsion | 13.685 | 14.510 |
| Non-1,4 VDW | -1.654 | -1.265 |
| 1,4 VDW | 15.989 | 17.480 |
| Charge/ Dipole | 10.334 | n/a |
| Dipole/Dipole | -3.848 | -4.452 |
| Total Energy | 44.124 | 40.477 |
From the above the resutls it can be seen that the product of the methylation is at a lower energy than the starting material this is due to the abscene of the formal charge in the system therefore reducing the energy of the products and making the reaction a spontaeous and thermodynamicaly favoured one. As is stated in the literature [1] the R and MgI will add in symltanious fashion in a 1,4 fasion slectivly, because the most stable state will only allow be permited
When the Methyl Magnesium Iodide is included in the starting system an error message appears saying "WARNING! No atom type was assigned to the selected atom."
Addition of NHPh
In this section the effect of adding NHPh to the molecule above was investigated and the results were seen as below and the energies of the moleuces minimised using MM2.
| Energy contribuition | Energy of precurser kcal/mol | Energies of products kcal/mol |
|---|---|---|
| Strech | 4.037 | 4.090 |
| Bend | 12.057 | 19.560 |
| Strech-Bend | 0.418 | 0.808 |
| Torsion | 9.081 | 6.109 |
| Non-1,4 VDW | 4.378 | 2.913 |
| 1,4 VDW | 29.391 | 34.034 |
| Charge/ Dipole | 34.034 | n/a |
| Dipole/Dipole | -4.883 | -5.952 |
| Total Energy | 63.541 | 61.5614 |
From the above results it can be seen that the products are more stable than the reactants and thus an exothermic reaction has occoured. It can also be said that there the main reason for the decrease in energy in the molecule is due to the fact that there is no formal charge. IT can also be seen here again that the carbonyl is determining the oreintation of the final product, here again maily due to the fact the carbonyl will direct the addition of the MeMgI in a 1-4 fashion as described in the literature[2].
Steriochemical and reactivity control of an Intermediate in the Synthesis of Taxol
It can be seen from the results that the second of the two moleucles is more stable as can be seen from the releitive energies 45.4467 kcal/mol (position a) and 43.089 kcal/mol (position b) respectively. The reason for this is the fact that there is a bycyclic systen substantially stabalises bridgehead olefins has the effect of lowering the reactivity. This can be explained by the ofefin strain values, as these are usually negative thus reducing the thermodynamic drive for the reaction[3]. It can also be said that when the carbonyl group is orteintated such as in postion a, that it forces the two rings down. This greatly incraeses the sterics clashes of the molecule and therefore greatly destabelises the molecule compared to b in which the sterics of the two rings are comparitively reduced and the reduced reactivity can be assigned to the high degree of sterics.
| | | | |
In order to obtain a structure which is futher optimised a program such as gausian should be used which would optimsie the structure futher, my taking into accourt the presence of differnt structural isomers of the chair boat and twist.
Modelling Using Semi-empirical Molecular Orbital Theory
In this section the moleucle structure of 9-chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene was first of all optimised by MM2 followed by Mopac (using MP6) with the symetry Cs assigned (using Gausian) it was finaly submited to SCAN to run the gausian log file. The same process was used for the monohydrated corresponding alkene in which the double bond remaining was syn to the C-Cl (although the point group was not assigned).
Regioselective Addition of Dichlorocarbene
On studdying the addtion of Dichlorocarbene to Halton et al[4] found that there was a highly selective addition across the double bond syn to the C-Cl. This can be said to be atibuted to the stabilisation of the C-Cl σ* with the anti-alkene therefore reducing the electron density of the anti-alkene compared to the syn-alkene, therefore making the syn-alkene more suseptable to electrophilic attack, as stated by Rzepa et al[5].
| HOMO-1 | HOMO | ||
|---|---|---|---|
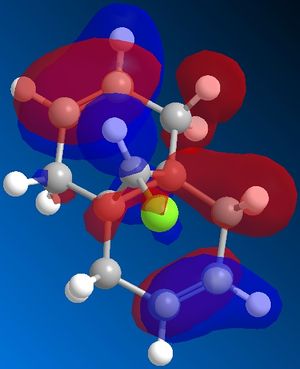 |
 | ||
| LUMO | LUMO+1 | LUMO+2 | |
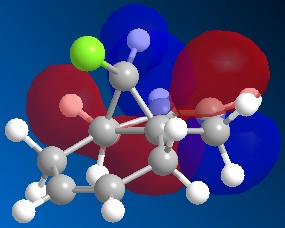 |
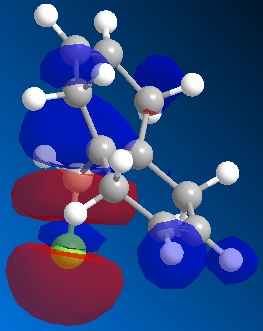 |
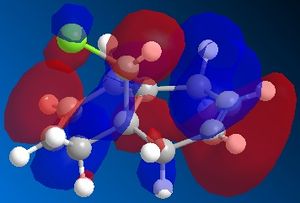 |
From the moleucular orbitals it may be said that the the above reasoning for the regiochemistry is justified. It can be seen from HOMO that there is more electron density on the syn-alkene and therefore that this is infact more electron dense. It can also be said that the the C-Cl σ* orbital is in the LUMO+1 and the stabilising π orbital of the anti-alkene is in the HOMO-1. NB it can be seen that there is a descrapancy with Rzepa et al here as the C-Cl σ*is part of the LUMO+2 but here is the LUMO+1. This will be due to the differnt type of calcuation sued (Rzepa et al used the PM3 instead of PM6) and differnt absolute conformations of moleucles used at the start of the minimisation process.
The Influence of the C-Cl bond on the vibrational frequencies of the molecule
In this section the resultant files from SCAN were opened in gausian were examined and the vibation frequencies of the monoand di alkene investigated.
| Type of strech | C-Cl | C=C anti to C-Cl | C=C syn to C-Cl |
|---|---|---|---|
| Dialkene[6] | 770.19 | 1737.06 | 1757.38 |
| Monoalkene[6] | 774.95 | n/a | 1758.05 |
It can be said from the above table that there is good corrolation with with literature, for the streching of the C-Cl bond [7].. However it can be seen that there are large deviations between the literature[8] (1620-1680), and the calculated C=C stretechs. This may be due to the stabilisation of the π orbital anti to the σ * C-Cl. When the syn and anti akenes are compared it can be seen that the di alkene's streches as described above are all higher than the corresponding dialkene it can also be said that the syn energies are higher than the corresponding anti alkenes. This implies a higher energy of the syn alkene which is therefore is at odds with the MO predicted reaction. It may be said that the caluclations therefore may have limitations.
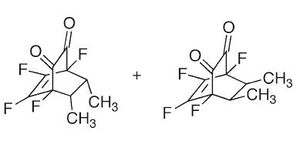
Mini Project-the study of the adduct product of o-Fluoranil products of its deils alder products
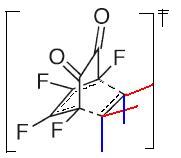
In this setion the the C13 NMR will be computed and the mechanism explored, of the products (being endo cis σ-diketone and exo cis σ-diketone) of the reaction of but-2-ene with o-Fluoranil and compared to the literature[9] [10], nb the adduct process will not be explored as this has no influence upon the isomer selectivity and is used as a means of seperation
Mechanistic and Orbital studdy
| reaction scheme |  | ||||||||||||
| reaction mechanism |  |
 |
 |
 |
|
 |
 |
and |  |
From the above mechanism it can be seen that the reaction may proceed via the endo or exo pathway, it can be seen from the bellow calcualtions show that the thermodynamicaly favoured product is the exo product, 59.772kcal/mol over the endo product, 59.623kcal/mol (nb caclulated using MM2). This is, as stated in the literature, the reverse of what would normally be expected of a deils alder reaction. The moleuclar orbitals (HOMO-1 to LUMO+2) were then calcualted with the same method as in previous part to try and explain the deviation from the expected product configuration. This it may be said is due to the delocalisation in general of electron density, as can be seen from the molecular orbitals below, particually in the case of the HOMO there is overlap of a pi nature between the pi c=c and the sigma c-c bond between thefore stabilisng the exo over the endo. It can also be said that the endo product would be normally be expected to form because of the fact that there will be secondar orbital interactions in the transition state which will be present in the endo and not the exo, namly the stabilisation of the LUMO orbital of the quninone with the HOMO of the cis but-2-ene, which is antibonding in nature, therefore increasing the stability of the endo trasition state over the exo and therefore favoring the product to be endo.
| endo |  |
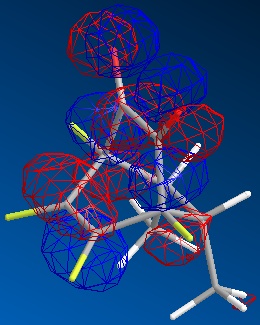 |
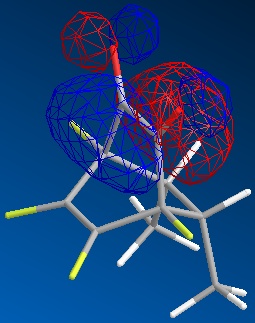 |
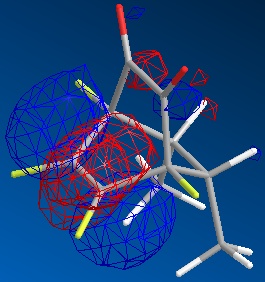 |
 |
| exo | 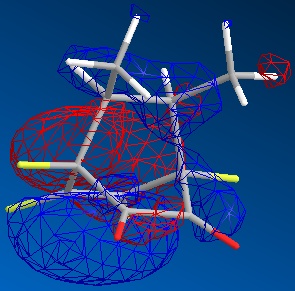 |
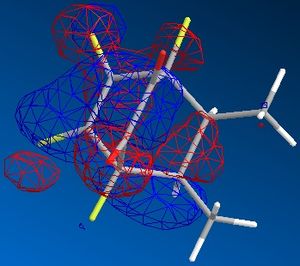 |
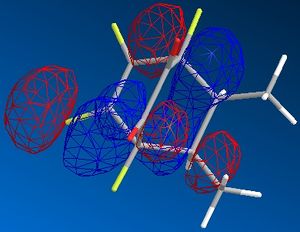 |
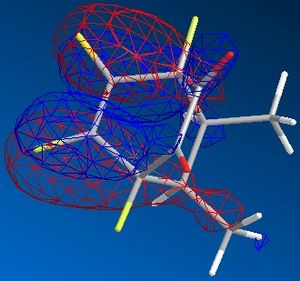 |
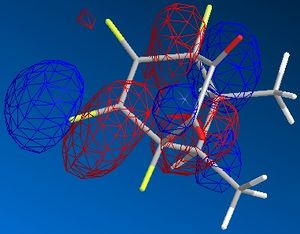 |
NMR analysis
In this section the NMR of the endo and exo adduct products were calculated though gaussian. In order to provide products of high enough quality for NMR the endo and exo product had to be reacted to give the final products of endo,cis- and exo,cis-1,4-Dihydro-1,2,3,4-tetrafluoro-11,12-dimethyl-1,4-ethanophenazine which were then examined by NMR.
| Endo assignment atoms | Exo assignment atoms |
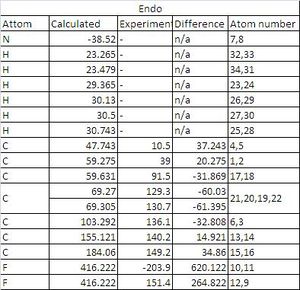 |
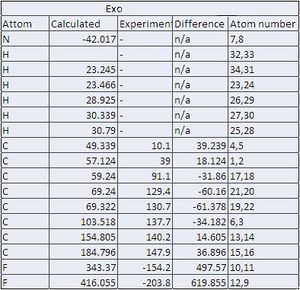 |
| Endo NMR | Exo NMR |
| Endo Literature NMR | Exo Literature NMR |
From the above table it can be seen that there is a varying degree of correlation between the experimental and literature of the C13 NMR and the 19F NMR data is wrong. It can also be said that there is only a negliable difference between the endo and the exo product and therefore that the NMR techneique is not ideal for identification. It could be recomended that melting points of the products could be used to identify the isomer before NMR or seperation tequniques such as the chromotography process described in the literature be used.
References
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838
- ↑ Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928
- ↑ Maier, W. F.; Schleyer, P. v. R., J. Am. Chem. Soc. 1981, 103, 1891-1900
- ↑ Brian Halton and Sarah G. G. Russell J. Org. Chem. 1991,56,5553-5556>Brian Halton and Sarah G. G. Russell J. Org. Chem. 1991,56,5553-5556
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447
- ↑ 6.0 6.1 }}>DOI:10042/to-2482
- ↑ . Socrates, Infrared and Raman Characteristic Group Frequencies, 3rd Edition, 2001, p. 65
- ↑ J. Coates, Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry, John Wiley & Sons Ltd, Chichester, 2000
- ↑ David M. Lemal, Dayong Sang and Sudharsanam Ramanathan J. Org. Chem., 2009, 74 (20), pp 7804–7811
- ↑ http://pubs.acs.org/doi/suppl/10.1021/jo9015562/suppl_file/jo9015562_si_001.pdf