Rep:Mod1:ch1508
Module 1: The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Ciaran Healy, March 2011
Introduction
The following page deals with a series of investigations into aspects of organic structure and reactivity. These are carried with the use of a variety of computational techniques. These begin with modelling molecular mechanics using the MM2 force field[1], which provides a simple model based around classical mechanical simulation of the bonds making up a molecule. Simple quantum mechanics can also be used, with methods such as MOPAC PM6 (used below)[2]. These semi-empirical calculations give a model of molecular orbitals, which can be used to rationalise various phenomina. Finally, such techniques will be applied to a particular problem in organic synthesis.
Modelling Using Molecular Mechanics
Hydrogenation of Cyclopentadiene Dimer
Cyclodimerisation of Cyclopentadiene
Cyclopentadiene undergoes dimerisation, via a [4+2] Diels Alder process (essentially one cyclopentadiene molecule is acting as the diene, and the other is acting as the dieneophile). There are two different possible isomers resulting from this Diels-Alder reaction between two molecules of cyclopentadiene.These are known as endo- and exo- products. The endo product sees the molecule folded back onto itself, giving a somewhat strange appearance. The alternative arrangement sees the molecule taking what perhaps might be the more expected shape.
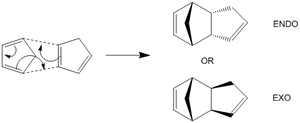
It would appear that the endo product might be unfavourable (relative to the exo version) due to the strange twisting that can be seen. One way to test this hypothesis is by using molecular modelling. Molecular mechanics can tell us which of the two products is the more strained, and hence which of the two we would expect to be the thermodynamically favoured product.
MM2 Molecular dynamics were used to investigate the two different forms, the total energy was recorded in both cases. The endo dimer gave a total energy value of 34.00 kcal/mol, whereas the exo dimer gave a total energy value of just 31.88 kcal/mol. The fact that this energy value is notably lower shows that this product is intrinsically more stable, and is therefore thermodynamically favoured.
Experimentally however, the endo product generally predominates. This means that the reaction must be under kinetic rather than thermodynamic control. The reason for this is that, as the molecules approach one another, their respective orientations are determined by interactions between the orbitals of the two cyclopentadiene molecules. This can be viewed in terms of frontier orbitals [3].
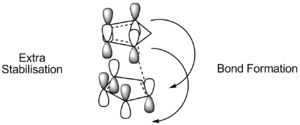
Determining the Position of Hydrogenation
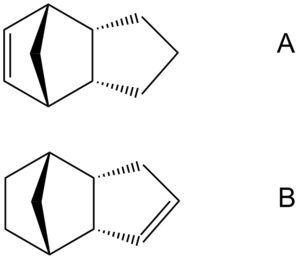
Given that we now know that the endo form is the more likely to be produced in the dimerisation, we can begin to determine what the product of the hydrogenation is going to be. There are essentially two different sites where the hydrogenation can take place (across the two carbon double bonds), which means that we have two possible products. The accessability of the two bonds is unlikely to be a factor, since both should be reasonably open to attack. Combining this with the fact that we know that the reaction takes a long time to complete, it makes sense to assume that the reaction is under thermodynamic control. This means that we can use molecular modelling to determine which product will be favoured (by looking at the total energy obtained via MM2 minimisation). The energies obtained, and the various component energies which make them up are shown in the table below. Additionally, a diagram (along with Jmols) is provided, so that the two different products (A and B) can be clearly identified.
| Energy (kcal/mol) | Product A | Product B |
|---|---|---|
| Stretch | 1.2761 | 1.0998 |
| Bend | 19.8332 | 14.5315 |
| Stretch-Bend | -0.8341 | -0.5460 |
| Torsion | 10.8221 | 12.5068 |
| Non-1,4 VDW | -1.2033 | -1.0720 |
| 1,4 VDW | 5.6421 | 4.5006 |
| Dipole/Dipole | 0.1621 | 0.1407 |
| Total | 35.6981 | 31.1614 |
From this data it can be seen that the energy of product B is significantly lower. We would therefore expect this to be the major product. The data (along with some chemical intution) also alows us to gain an insight into why this product is the more favourable. This can be acheived by analysing the breakdown of contributors to the overall energy of each molecule. The biggest difference between the two sets of data is the bend value, with a difference of over 4.3 kcal/mol.
The explaination for this difference can be arrived at by consideration of the environment in which each double bond can be found. Both double bonds make up part of a ring system, their presence is likely to add some ring strain (they mean that the other carbons in the ring are unable to have ideal geometry). We can identify the norborene unit as being the more strained, due to the presence of the methylene bridging unit. This means that hydrogenating at the double bond in the norbene unit will release more strain than hydrogenating that contained in the simpler five-membered-ring will.
This effect can be demonstrated by calculating the bond angles at the sp2 carbons in the endo dimer. For the carbon-carbon double bond in the norborene unit, this angle is 108°, for the other double bond, 112°. These are both a little way from the ideal 120° for a carbon with sp2 hybridisation, but the norborene double bond has an angle which is very close to (and infact even lower than) the ideal sp3, tetrahedral, bond angle of 109°. This means that it should be relatively easier to hydrogenate at the norborene unit (giving product B) than at the other site(giving product A). This means product B should be favoured.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).
In this section, the stereoselectivity of two different nucleophillic additions to a pyridinium ring present in (NAD+). The first is an attack using a Grignard reagent, the second uses aniline. Both these reactions exhibit a high degree of stereoselectivity, this selectivity can be investigated and explained using molecular modelling.
Attack by a Grignard
In this reaction, a proline derviative is reacted with a simple Grignard reagent (MeMgI). This reaction is highly regio and stereoselective. This is, in large measure, due to the manner in which Grignard reagents coordinate to the oxygen of the carboyl unit [4]. The mechanism by which the reaction proceeds can be seen in the diagram below.
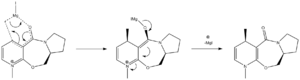
Here it can be seen that once the Grignard has coordinated to the carbonyl, the methyl group can be added in a conjugate fashion. The delivery of the methyl group might therefore be directed by the orientation of the C=O unit, relative to the plane of the pyridine ring. MM2 energy minimisation can be used to find the preferred conformer (that with the lowest energy), the dihedral angle between the carbonyl and the planar ring can then be obtained, and the result used to better understand the reaction taking place.
The carbonyl carbon is, as are the carbons in the aromatic ring, sp2 hybridised, this means that the ideal structure for this part of the molecule in isolation would be planar, however, the shape of the rest of the molecule forces this to change. After extensive manipulation, the lowest energy conformer that could be obtained was recorded with a total energy of 42.77kcal/mol. This conformation had a dihedral angle of 11° above the plane of the ring. Other relatively stable conformers were found, but all had the C=O angle above the plane of the ring (one example having a bond angle of 20° with an energy of 43.76kcal/mol). The lowest energy conformation for the proline derivative starting material can be seen in Jmol form by clicking the button below.
11° might not seem like a particularly significant angle, however this is likely to be enough to ensure that the addition is directed onto the top side of the ring. This explains the stereoselectivity of the reaction.
It is worth noting that the Grignard itself cannot be modelling using MM2, since no parameters for Mg exist.
Attack by Aniline
The nucleophillic attack by aniline on a similar ring system gave a similar result, in so far as the added group (in this case -NHPh) is added above the ring.
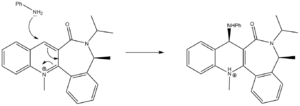
In an attempt to explain this fact, a similar process as before was carried out, with the carbonyl adjacent to the point of attack of particular interest. The structure was subjected to energy minimisation using MM2. Similar considerations (about the carbonyl in isolation being stable when planar with the ring) to those discussed above apply here. The difference is that the rest of the molecules forces the distorion of the carbonyl in a different direction. After a long period of manipulation, the preferred conformer was settled upon, with the dihedral angle of the carbonyl to the planar aromatic system found to be 20° below the plane of the ring. The total energy of this conformation was found to be 62.63kcal/mol. A number of attempts to generate a stable conformation with the carbonyl above the ring were made, but none proved successful. The conformation settled upon for the starting material can be seen in Jmol form by clicking the button below.
The fact that the oxygen here lies below the ring suggests that unlike in the previous example, there is no coordination at work here, but a simpler explaination for the selectivity observed, sterics. The attack proceeds by utilising the lone pair of the nitrogen. This lone pair can be expected to have significant repulsions with other lone pairs, for example those of the carbonyl oxygen. These lone pairs seem to lie close to the Bürgi-Dunitz angle on the bottom face, which would make attacking there extremely difficult. This explains why aniline only attacks on the top face, rather than been drawn to the top (in the way that the Grignard above is), it is repelled from the bottom face making the top face the only option.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
An important intermediate in the synthesis of taxol is shown below[5]. It exhibits atropisomerism, and the two isomers (A and B) are shown below. Atropisomerism occurs when the rotation about a single bond becomes restricted, often because of steric barriers. It is known that when the mixture of both isomers is left to stand, it all converts to one form (the more stable isomer). In order to determine which of the isomers this is, molecular modelling can be used. Here MM2 was used again, along with MMFF94. The energies obtained were as shown in the table below.
| Energy (kcal/mol) | Isomer A | Isomer B |
|---|---|---|
| Stretch | 2.5169 | 2.5466 |
| Bend | 10.9587 | 10.7249 |
| Stretch-Bend | 0.3325 | 0.3152 |
| Torsion | 20.9502 | 19.8054 |
| Non-1,4 VDW | -1.9116 | -1.4425 |
| 1,4 VDW | 13.5714 | 12.5600 |
| Dipole/Dipole | 0.1156 | -0.1807 |
| Total MM2 | 46.5338 | 44.3289 |
| Total MMFF94 | 63.9241 | 60.5547 |

The first and most obvious conclusion to draw here is that isomer B is the more stable. This is the isomer with the carbonyl pointing down. This fact can be rationalised by looking at the shape of the six-membered-ring at one end of the molecule. When the carbonyl points up, this is in a rather unstable arrangement (a twist-boat shape); when the carbonyl is pointing down, this is in a much more stable arrangement (a fairly regular chair). This difference in the shape of the ring clearly has a considerable effect on the overall energy of the molecule.
You can also see that the two methods produce essentially the same result. This does not apply to the absolute values (which are not directly comparable from method to method). However, both methods give a result where isomer A is a little higher in energy than isomer B. In each case the difference between the isomers is roughly 5% of the lower value (4.97% for MM2 and 5.56% for MMFF94).
The isomers have been reported to react unusually slowly. The explaination for this is that both isomers are examples of hyperstable alkenes [6]. This is due to the presence of a bridgehead carbon-carbon double bond incorporated into the ring system. This consideration only applies in a ring system of a suitable size [7], where the alkene has less strain than the coresponding alkane. This stability makes compounds of this type very slow to react.
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
Explaining Regioselectivity
9-chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene has been shown to have rather regiospecific reactions with appropriate reagents. One such reagent is dichlorocarbene. With dichlorocarbene the syn adduct is preferentially formed ahead of the diadduct, i.e. only one side of the molecule sees any reaction take place[8]. It was reported that the ratio of syn- and di- adducts was in excess of 3:1 in favour of syn- (72:23).
This selectivity is difficult to fully understand using a simple mechanical model (e.g. MM2), to get around this we need to use a method that takes account of molecular orbitals. MOPAC/PM6, which gives a good approximation of the valence-electron molecular wavefunction (most importantly the HOMO), is a useful technique in this regard. We would expect the HOMO to be very important in determining reactivity.
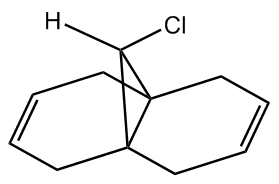
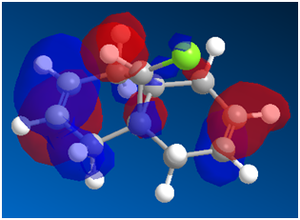
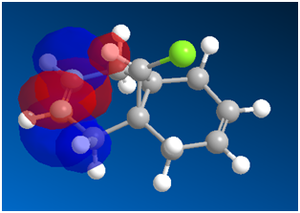
Using a MOPAC/PM6,the following MOs were obtained:
 |
 |
 |
 |
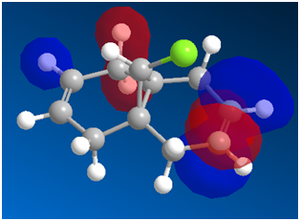 |
Looking at the HOMO first, one thing is striking. There is a much greater concentration of electron density on the syn- side of the molecule. This fits well with the experimental data: dichlorocarbene is an electrophile, so one would expect that it will react preferentially on the side of the molecule with more electron density. One important reason that some diadduct is produced is that in the HOMO-1 and LUMO MOs, the majority of the electron density is on the anti- side of the molecule. This means that under unusual conditions, excitation, etc. it is possible for the carbene to attack the other side of the molecule.
It has also been shown that there is an antiperiplanar interaction between the C-Cl σ* orbital (in LUMO+1) and the occupied π orbital on the exo side (in HOMO-1). This provides an important stabilisation of 0.08eV compared to the expected endo form. This helps the diadduct to compete to a significant extent, giving the large amount of minor product that we see[9].
Vibrational Analysis
Analysing the vibrations of the molecule, and its monoalkene derivative gives the following results:
| Derivative | Syn-alkene stretch | Anti-alkene stretch | C-Cl stretch |
|---|---|---|---|
| Dialkene (12) | 1755 | 1738 | 772 |
| Hydrogenated monoalkene (syn-alkene) | 1758 | n/a | 774 |
One way of interpreting the values shown, is in terms of bond strength (where higher wavenumber values are indicative of stronger bonds). The C-Cl frequency is roughly the same in both cases, about C-Cl 772cm-1 and 774cm-1. This is value is not unusual, indicating that the C-Cl bond here is of normal strength[10]. Looking at the C=C bonds in the dialkene, one can see that there is a difference of 17cm-1, suggesting that one bond will be a little stronger than the other. Specifically that the syn-alkene will be more stable than the anti-. It should be noted that both bonds are at a higher energy than is normal (1640-1680cm-1[10]). The syn-alkene stretch is particularly higher, this seems to be the more stable, which fits with the MO picture seen previously, where there was a real difference in reactivity for the two C=C bonds. The removal of the anti-alkene, seems to have little effect on the other two stretches being studied.
Structure based Mini project using DFT-based Molecular orbital methods
The reaction being investigated here is a Lewis Acid catalysed Diels-Alder reaction between cyclopentadiene and methylcrotonate[11]. The catalyst here is: N-trimethylsilyl bis(trifluoromethanesulphonyl)imide, referred to as TMSNTf2. The exact way in which this catalyst works in not of that much importance here. Essentially, it enhances suitable dienophiles by activation of their carbonyl group with an electron withdrawing group. This obviously helps to accelerate the Diels-Alder reaction.
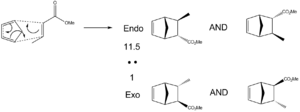
As can be seen there are four distinct possible products. These fall into two pairs of diastereoisomers, one endo (the major product) and one exo (the minor product). It is difficult to distinguish between the two forms of each cycloadduct (endo/exo), for example, they will give the same NMR spectra. A major aim of this particular mini-project will be to study the NMR data provided in the paper reporting this synthesis; and, by utilising computational techniques, to add further depth in whatever way possible. One obvious example of how this might be done, would be to attempt to assign the various 13C NMR peaks to the correct carbon atoms. This has not been done in the source. By accurately determining the 13C NMR using computational tools, which carbon atoms go with which real peaks might be identified. Since the NMR spectra of the two forms of each adduct will be the same, for the purposes of NMR investigation, the diastereoisomerism will be ignored (meaning we only need to worry about endo- and exo- forms. Optical rotation could be calculated for the various possible forms. However, this is a complex, lengthy process, which can give rather variable results, so will not be undertaken here, where time and resources are limited.
In the forthcoming assignments of the 13C NMR spectra, the carbon atoms will be numbered as shown in the diagram below.
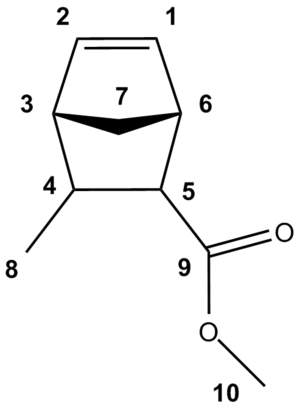
13C NMR Spectrum of Endo Cycloadduct
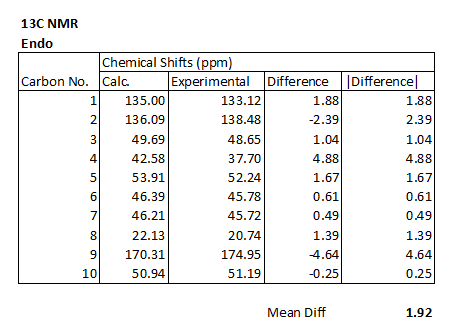
The following table shows the calculated values for the chemical shifts, and the experimentally obtained ones. It also shows the difference between them.
This data (difference between calculated and experimental chemical shifts for each C atom) is represented in the following graph.
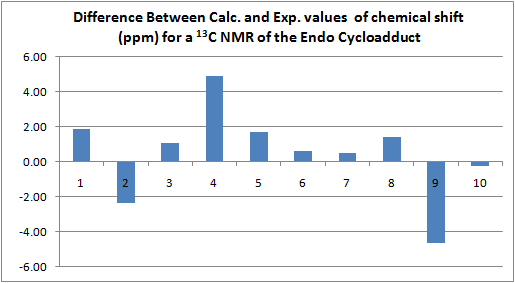
The table also contains the assignments that have been made with the use of 13C NMR prediction.
The biggest of the discrepancies between calculated and experimental data is found for the carbon which is bound to two oxygen atoms (carbon 9). This carbon is unsurprisingly the most deshielded due to the two electronegative carbons drawing elctron density away. The reason a difference is observed may well be to do with the basis set being used, it may not handle this sort of situation particularly well (this is backed up by the fact that this issue is seen for both of the spectra). As far as assigning this peak goes, the variation does not pose a huge problem, since there are no other peaks within roughly 35ppm of this one in either the real of calculated spectra.
13C NMR Spectrum of Exo Cycloadduct
The following table shows the calculated values for the chemical shifts, and the experimentally obtained ones. It also shows the difference between them.
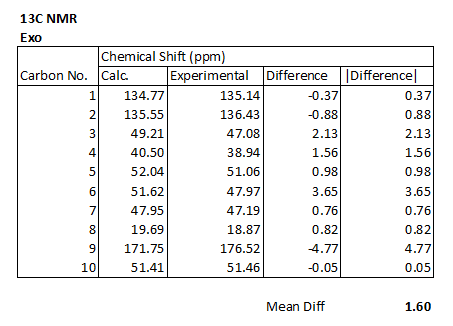
The table also contains the assignments that have been made with the use of 13C NMR prediction.
This data (as far as the differences for each carbon are concerned) is represented in the following graph.
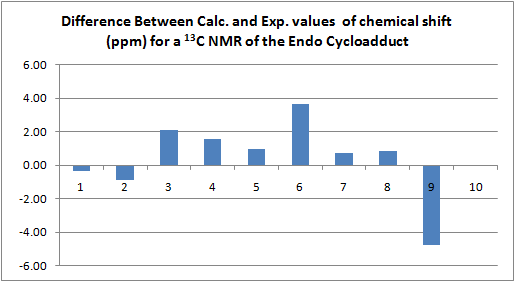
As discussed above problems were encountered in the precise calculation of the chemical shift for carbon 9, but this does not stop us from accurately assigning it.
Distinguishing between the two isomers using 13C NMR Spectra
The two 13C spectra are largely rather similar, making it difficult to discern any obvious difference, that might help one conclusivly identify which cycloadduct was which given a pair of unmarked samples. However on closer examination, there is one interesting difference in experimental data, which might provide a manner of differentiation. Looking at carbons 1 and 2 (either end of the C=C bond, you can see that there is a larger gap between the two value for the endo adduct (over 5ppm between them), for the exo form, this is much lower (with a gap of less than 1.5ppm). This suggests that the endo form contains a much more polarised C=C bond than the exo. The reason for this must be to do with the changing (up/down) postion of the CO2Me unit, and its varying ability to draw electron density away.
This is not picked up in the calculated spectra. The most likely reason for this is that there is another possible conformer of the enod product, of a similar energy, where there is better orbital overlap, towards the C=C area at the other end of the molecule, which would make this drawing of electrons considerably more effective. This would most likely involve rotation around the bond linking carbons 5 and 9. It might be interesting
It could also be that this is related to the problems described earlier with the problems in accurately determining the chemical shift of carbon 9. The two problems might well share the same root.
Published 13C NMR Spectra
Endo: http://hdl.handle.net/10042/to-7443
Exo: http://hdl.handle.net/10042/to-7445
Other methods of distinguishing between the two isomers
It appears from the literature that the 1H NMR spectra of the two products are rather different in some ways (particularly multiplicities for some of the lower shift protons). However, this sort of spectrum is difficult to compute, so will not receive a great deal of attention. One intersting spectroscopic possiblity, with regards to making a clear differentiation between the two is NOESY.
NOESY is basen on the nuclear Overhauser effect (NOE), which is based on the fact that proton which are close together in space can still influence one anothers' signals, even if they are some distance apart in terms of bonds. Suitable protons seem to exist here, and this was checked using Janocchio, which can estimate the NOE between two protons.
The protons on the underside of the ring system are fixed in positions relatively close to one another, while in some cases being four bonds apart. which protons are in position to participate depends on which groups are up/down, i.e. which isomer a molecule is.
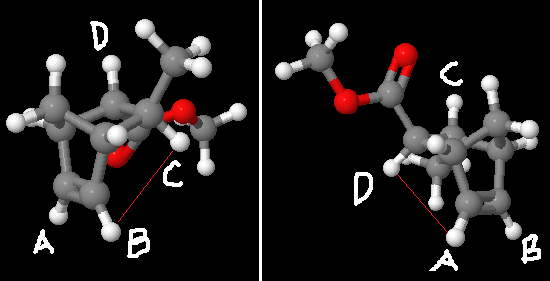
It can be seen that in the endo adduct protons B and C might be suitable for NOESY analysis, and in the exo adduct A and D might be suitable.
To check this, Janocchio was used to estimate the size of the interactions that might be seen. These were found to be small, and of a similar magnitude in the two cases. For endo (B to C), the NOE value estimated was -0.0106. For exo (A to D) the NOE value was given as -0.0099. With no values literature to compare to here, it is difficult to determine how accurate these calculations are, but they do suggest that NOESY might be applicable and useful to other molecules of this sort to aid determination between exo and endo isomers.
Conclusions
Having completed the above investigations, the usefulness of computantional techniques to synthetic chemists is obvious. In addition to rationalising the porducts from reactions themselves, their spectra can be simulated, investigated and generally better understood. There are serious limitations, for example the inability to accurately simulate 1H NMR spectra. However, it is surely only a matter of time before a suitable system is developed - such is the speed of development in increasing computing power, and computational chemists' increasing understanding of what is possible. Even so, the tools we have today are incredibly useful in predicting, understanding and more effectivly analysis complex reactions.
References
- ↑ N. L. Allinger, J. Am. Chem. Soc., 1977, 99, 8127.
- ↑ Molecular Modelling: Principles and Applications, A. R. Leach, 1996.
- ↑ Clayden et al., Organic Chemistry, 2001, p916
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ # S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ Maier, W. F.; Schleyer, P. V. R, J. Am. Chem. Soc., 1981, 103, 1891.
- ↑ JS Kim, Bull. Korean Chem. Soc., 1997, Vol. 18, No. 5
- ↑ B. Halton and S. G. G. Russell, J. Org. Chem., 1991, 56, 5553-5556.DOI:10.1021/jo00019a015
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ 10.0 10.1 IR Correlation Table[1]
- ↑ B. Mathieu, L. Ghosez, Tetrahedron, 2002, 58, 8219-8226
