Rep:Mod1:albert
The Hydrogenation of Cyclopentadiene Dimer
Dimerisation of Cyclopentadiene
From ChemBio3D, the total energy recorded for the exo dimer is 31.8818 kcal/mol while for the endo dimer, the total energy recorded is 34.0089 kcal/mol. Comparing both energies, the exo dimer is considered to be thermodynamically more stable than the endo dimer due to a smaller total energy. However, as cyclopentadiene dimerises rapidly at room temperature [1], the Diels-Alder reaction here would therefore be irreversible thus favouring the kinetically stable endo dimer over the exo dimer.
Hydrogenation of dicyclopentadiene

| Properties | Product 3 | Product 4 |
|---|---|---|
| Stretch | 1.2659 | 1.0096 |
| Bend | 19.8063 | 14.4971 |
| Torsion | 10.8698 | 12.5012 |
| Hydrogen Bonding | 0.1621 | 0.1405 |
| Van Der Waals (VDW) | 5.6394 | 4.5226 |
| Total Energy (kcal/mol) | 35.6953 | 31.1551 |
The stretch refers to the energy associated with distorting bonds from their optimal length while the bend refers to the energy associated with deforming bond angles from their optimal values. From table 1, product 4 yields a lower stretch, bend and intermolecular forces of interaction than product 3. With lower intermolecular forces of interaction for product 3 than product 4 it therefore suggests that product 3 is thermodynamically more stable than product 4 as its total energy is lower. As such the hydrogenation of the endo dimer is thermodynamically controlled.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
N-Methyl Pyridoxazepinone (5)
'''N-Methyl Pyridoxazepinone (5)''' |
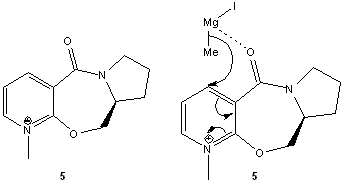
From literature [2] the use of the Grignard reagent, MeMgI results in a highly selective product. Using Chem Bio 3D, the interaction betweeen the Grignard reagent and 5 resulted in an unexpected error probably due to the absence of the element Mg in the programme. To obtain the lowest optimisation energy possible, the lone pairs on the oxygen ring was positioned away from the carbonyl carbon of the amide fuctional group as well as placing the adjacent hydrogen atoms as far as possible from the carbonyl oxygen to minimise intramolecular hydrogen bonding.
Considering the geometry of the carbonyl group with respect to the aromatic ring (i.e. the NR-pyridine), an optimal dihedral angle of 24.00o was obtained (see graph below) which allows for a favourable coordination between the electropositive magnesium of the Grignard reagent and the amide oxygen to result in the methyl group to attack the carbon-4 position of the aromatic ring via an intramolecular reaction involving a 6-membered transition state as supported by literature [2]. The dihedral angle obtained also suggests that the carbonyl group should be at a different plane from the carbon-4 of the aromatic ring to minimise the effects of sterics in the Grignard reaction. As the reaction mechanism depends on the relative syn position of the carbon-4 of the aromatic ring and the carbonyl group, perhaps a ring-locking substituent such as tert-butyl could replace the methyl-substituent on the nitrogen atom on the aromatic ring.
The plot below shows the relationship between the total energy of the molecule with respect to varying the dihedral angle using Chem Bio 3D.
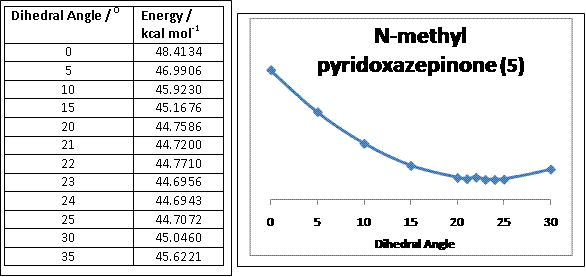
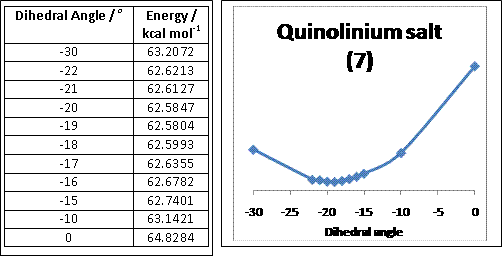
Quinolinium salt (7)
'''Quinolinium salt (7)''' |
Using Chem Bio 3D, the optimal dihedral angle between carbon-4 of the aromatic pyridinium ring and the carbonyl group for molecule 7 was -19.00o or 199.00o (see graph below). This suggests that by having the carbonyl group in an anti relationship with that of carbon-4 on the aromatic ring it favours an unhindered nucleophilic attack on carbon-4 of the aromatic pyridinium ring minimising any competing reactions from the adjacent carbonyl carbon. In order to obtain the lowest optimisation energy possible, the methyl and iPr substituents were as far as possible from the benzene substituents. Using Chem Bio 3D, the addition of aniline (or PhNH2) to 7 resulted in a much lower optimisation energy when the phenyl-group of aniline was placed as close to carbon-4 of the aromatic pyridinium ring and the phenyl-group placed away from the bulky substituents on 7.
In order to improve the molecule, Gaussian can be used to obtain the optimised energy through the SCAN option instead of using the MM2 function.
The plot below shows the relationship between the total energy of the molecule with respect to varying the dihedral angle using Chem Bio 3D.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol

Considering the retrosynthesis of Taxol [3] the carbonyl oxygen points at the back of the plane of the paper and as such in using Chem 3D Bio to model intermediates 10 and 11, the carbonyl oxygen, whether pointing upwards or downwards, has to be at the back of the plane of the paper to reflect the stereochemistry of Taxol.
Intermediate 10 adopts a twist-boat conformation with the carbonyl group pointing upwards. Although there exists a favourable stabilising σ-π* conjugation between the σC-H and π*C=O orbitals, however, there exists significant transannular strain between the carbonyl oxygen and the adjacent hydrogen atoms of similar upwards orientation resulting in a total energy of 54.5476 kcal/mol, which is higher than that of intermediate 11. For intermediate 11, it adopts a chair conformation with the carbonyl group pointing downwards. Although there is the absence of the stabilising σ-π* conjugation between the σC-H and π*C=O orbitals due to unfavourable orbital geometry as well as the slight presence of transannular strain between the hydrogen atoms pointing upwards despite its large size, the overall absence or minimisation of intramolecular interactions as observed from the values of 0.0438 for intermediate 11 as compared to 0.1805 for intermediate 10 obtained from Chem 3D Bio is a predominating factor that results in a lower total energy of 44.2834 kcal/mol for intermediate 11. As such, intermediate 11 is the more stable isomer.
From literature [4], bicyclic systems can provide substantial stability to bridgehead olefins. Hyperstable olefins have negative olefin strain (OS) energies which lowers the thermodynamic driving force of the reaction and the molecule with the lowest OS usually incorporates the trans-cycloalkene moiety in the larger ring. Olefin strain (OS) values correspond to how much extra strain the introduction of a double bond induces [5]. As such, the slow reaction with the tetra-substituted bridgehead alkene should be attributed to kinetic factors and more specifically, steric hindrance that inhibits electrophiles from an electrophilic attack on the alkene thus rendering the alkene to react slowly.
In order to further minimise the energy of the structure, it is suggested to use Gaussian instead of MM2 for a more accurate determination. Considering the cyclohexane conformation, the chair conformation is lowest in energy as compared to the twist-boat and it is suggested that the movement of atoms in the ring can take the chair conformation into consideration.
Regioselective Addition of Dichlorocarbene
Orbital Control of Reactivity
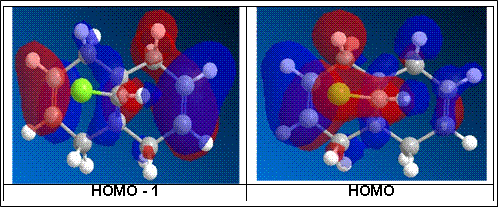
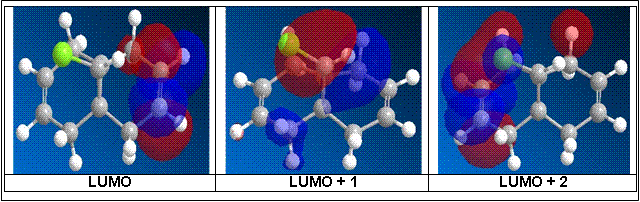
From literature [6] the calculations were performed under PM3 conditions while for this computational experiment, the calculations were performed under PM6 conditions resulting in some differences in the molecular orbital representation of the HOMOs and LUMOs from literature. However, what concurs with literature, is the molecular orbital representation of the HOMO for the computational experiment which therefore validates the results obtained from literature particularly on the selective reactivity of the alkenes.
From the HOMO or highest occupied molecular orbital of the dialkene, it shows a large electron cloud build up in one region (i.e. where the C=C bond is nearest to the C-Cl bond or the syn-alkene) over the other. The alkene bond is electron rich while the electronegative Cl atom has a strong inductive electron-withdrawing effect. The net effect of the electron-withdrawing nature of Cl as well as the electron rich nature of the alkene, will give rise to a spatial negative charge build up in the region of the syn-alkene as shown by the orbital interaction of the HOMO of the dialkene.
As such the more nucleophilic alkene is the syn-alkene and will as such be more susceptible to electrophilic reactions as supported by the orbital interactions of the LUMO of the dialkene as shown below. This therefore suggests that the anti-alkene is more electrophilic and hence is less susceptible to electrophilic reactions. This is supported by literature [6] where the σ*C-Cl interacts with the пC=C to result in a stabilising interaction that lowers the energy of the molecular orbitals to result in a comparatively more reactive syn-alkene.
Influence of C-Cl bond on the Vibrational Frequencies of (12) and (13)
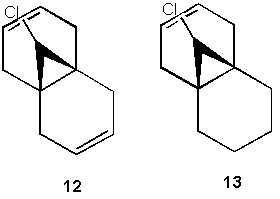
The stretching frequeny of C-Cl for both molecules 12 and 13 are 770.95 cm-1 and 779.80 cm-1 respectively which corresponds to the literature [7] stretching frequency of 780 cm-1. In determining the correct stretching frequency for the C-Cl bond using Gaussview 5.0, a closer scrutiny of the C-Cl bond is required to avoid taking the rocking motion of non-C-Cl bonds as the stretching motion of the C-Cl bond.
Comparing the IR spectrum of 12 DOI:10042/to-2480 and 13 DOI:10042/to-2481 , for 12, there are two peaks each due to one C=C bond where the stretching frequency of 1737.12cm-1 pertains to the anti-alkene and 1757.35cm-1 that pertains to the syn-alkene. For 13, there is only one peak that corresponds to the syn-alkene of stretching frequency 1753.80cm-1. From literature [7], the stretching frequency for C=C is 1620 - 1680 cm -1. The values obtained for the stretching frequency for the alkene bonds for both 12 and 13 are much higher than literature and corresponds to the literature [7] stretching frequency for C=O functional groups of 1670 - 1870 cm -1 instead. This higher stretching frequency reported for the alkenes present in both 12 and 13 could be due to the influence of the adjacent electronegative Cl atom.
Energy is proportional to frequency and the absence of the anti-alkene in 13 suggests that the anti-alkene is comparatively weaker than the syn-alkene and this is supported by the lower stretching frequency of the anti-alkene for 12. This observation however runs opposite to the molecular orbital representation of the dialkene where the syn-alkene was shown to be the more reactive alkene.
One possible explanation for this discrepancy could be that as Gaussian employs an empiral calculation model, it is only valid when the first derivatives of the energy with respect to the displacement of the atoms are zero [8]. Furthermore the method for calculation employed may also have its limitations which may give rise to a wholly different result.
Additional Reading
- For the synthesis of 12, refer to J. J. Sims and V. K. Honwad, J. Org. Chem., 1969, 34, 496 - 499. DOI:10.1021/jo01255a004
Structure based Mini Project using DFT-based Molecular Orbital Methods: Enantioselective Diels-Alder Reactions[9]
Introduction
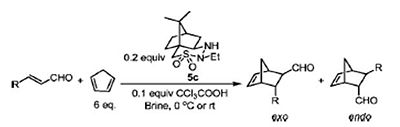
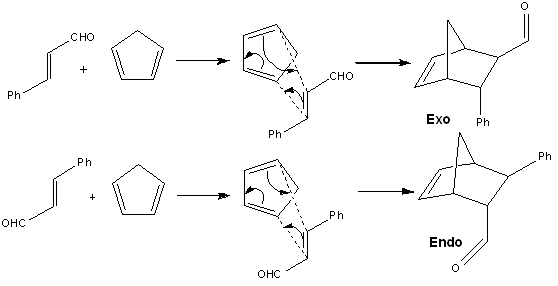
For this project, computational teachniques was employed to validate the authenticity and reliability of the results from a literature paper. In this paper [9], the enantioselective Diels-Alder reaction was performed using cyclic sulphonyl hydrazine (CaSH) as an organocatalyst together with trichloroacetic acid as a co-catalyst. The reaction scheme (extracted from literature [9]) is shown below:
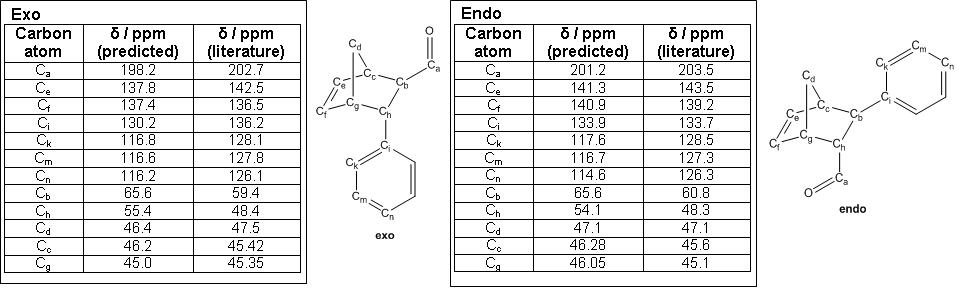
For the purpose of the project study, only R = Ph was considered so as to avoid a highly conformationally flexible molecule. From the Diels-Alder reaction, two products were obtained namely (1R,2R,3R,4S)-3-Phenylbicyclo[2,2,1]hept-5-ene-2-carboxaldehyde as the exo product and (1S,2R,3R,4R)-3-Phenylbicyclo[2,2,1]hept-5-ene-2-carboxaldehyde as the endo product.
13C NMR Analysis
From literature [9], for the exo product 13C NMR (100 MHz, CDCl3) δ 202.7, 142.5, 136.5, 136.2, 128.1, 127.8, 126.1, 59.4, 48.4, 47.5, 45.42 and 45.35. From the predicted 13C NMR for the exo product, 13C NMR δ 198.2, 137.8, 137.4, 130.2, 116.8, 116.2, 65.6, 55.4, 46.4, 46.2 and 45.0.
From literature [9], for the endo product 13C NMR (100 MHz, CDCl3) δ 203.5, 143.5, 139.2, 133.7, 128.5, 127.3, 126.3, 60.8, 48.3, 47.1, 45.6 and 45.1. From the predicted 13C NMR for the endo product, 13C NMR δ 201.2, 141.3, 140.9, 133.9, 117.6, 116.7, 114.6, 65.6, 54.1, 47.1, 46.28 and 46.05.
The table below shows the 13C NMR analysis for both exo and endo product.
Spectroscopically, the 13C NMR values obtained through computational prediction for both the exo DOI:10042/to-2513 and endo DOI:10042/to-2514 products correspond with that of literature. In addition, there is a large similarity in terms of the 13C NMR values between the endo and exo product. This suggests that an alternative method is required to seperate one enantiomer from another. Taking into account that from literature [9], the use of CaSH resulted in a ratio of 1:1.1 for exo:endo products with 78% exo ee and 93% endo ee, this meant that the exo and endo products were possible to separate out through physical methods such as chiral chromatography as described in the literature [9] for further analytical analysis. Using Gaussian, the optical rotation [α] at chloroform at 589nm for the exo product was -180.11o while that for the endo product was -84.69o suggesting that the use of optical rotation is another plausible means of identification for the exo and endo products which was not recorded in literature [9].
Mechanistic and Theoretical Analysis
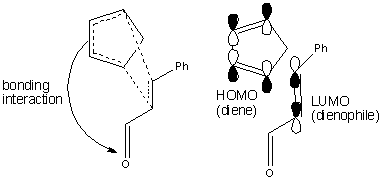
From literature [10], for irreversible Diels-Alder reactions, the endo diastereoisomer is often preferred over the exo because of kinetic factors. For this reaction, the preferred endo diastereoisomer has the carbonyl groups of the dienophile closest to the developing π bond at the back of the diene so that in the transition state, there exists a bonding interaction between the C=O group of the aldehyde and the back of the diene. In this Diels-Alder reaction, the LUMO of the electron-deficient dienophile due to the presence of the electron-withdrawing aldehyde substituent will interact favourably with the HOMO of the electron-rich diene thus favouring the endo product.
Both exo and endo products adopt the A1,3 elicpsing conformation to yield a favourable σC-H and π*C=O interactions and to minimise steric replusion between the oxygen lone pair on the carbonyl oxygen and the adjacent hydrogen atom on carbon-3 of the ring as shown in the molecular orbital representation above. Comparing the energies of the optimised exo and endo products from Gaussian, the endo product yields a smaller total energy suggesting that the orbital interaction between the σC-H and π*C=O is more stabilising for the endo product than it is for the exo product. This therefore suggests that for the endo product, there is a smaller energy gap between the σC-H and π*C=O orbitals and hence the endo product is favoured.
Conclusion
From this computational exercise, the results recorded in literature [9] corresponded to the results obtained from computational analysis as well as from theoretical analysis of the issue suggesting a pivotal role that computational chemistry can play in future research.
References and Citations
- ↑ Organic Synthesis: Cyclopentadiene http://www.orgsyn.org/orgsyn/prep.asp?prep=cv4p0238
- ↑ 2.0 2.1 A. G. Schultz and L. Flood, J. Org. Chem., 1986, 51, 838-841. DOI:10.1021/jo00356a016
- ↑ H. Kusama, R. Hara, S. Kawahara, T. Nishimori, H. Kashima, N. Nakamura, K. Morihira and I. Kuwajima, J. Am. Chem. Soc., 2000, 122, 3811-3820. DOI:10.1021/ja9939439
- ↑ A. B. McEwent and P.V.R. Schleyer, J. Am. Chem. SOC., 1986, 108, 3951-3960. DOI:10.1021/ja00274a016
- ↑ P. M. Warner, Chem. Rev., 1989, 898, 1067-1093. DOI:10.1021/cr00095a007
- ↑ 6.0 6.1 B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447 Cite error: Invalid
<ref>tag; name "HOMO LUMO" defined multiple times with different content - ↑ 7.0 7.1 7.2 G. Socrates, Infrared and Raman Characteristic Group Frequencies, Third Edition, 2001, Pg. 65
- ↑ J. W. Ochterski, Vibrational Analysis in Gaussian, 1999, http://www.gaussian.com/g_whitepap/vib.htm
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 H. He, B. J. Pei, H. H. Chou, T. Tian, W. H. Chan and A. W. M. Lee, Org. Letters, 2008, 10, 2421-2424 DOI:10.1021/ol8005826
- ↑ J. Clayden. N. Greeves, S. Warren and P. Wothers, Organic Chemistry, 2000, First Edition, Pg. 913