Rep:Mod1: Lukas Miseikis
The Hydrogenation of Cyclopentadiene Dimer
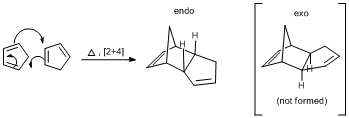
Cyclopentadiene dimerizes via thermal 4n + 2 cycloaddition (also known as Diels-Alder) reaction to give a fused bicyclic ring structure. It has been observed that the product of this conversion is exclusively endo but not the exo as shown below:
To account for this observation both endo and exo molecules were optimised using Molecular mechanics MM2 code (see figures 1 and 2)and results are presented as follows:
| Stretch | Bend | Stretch-Bend | Torsion | non 1-4 VDW | 1-4 VDW | Dipole-Dipole | Total energy | |
|---|---|---|---|---|---|---|---|---|
| Endo | 5.230 | 87.24 | -3.496 | 39.80 | -6.456 | 18.08 | 1.873 | 142.3 |
| Exo | 5.376 | 86.10 | -3.506 | 32.03 | -5.929 | 17.72 | 1.580 | 133.4 |
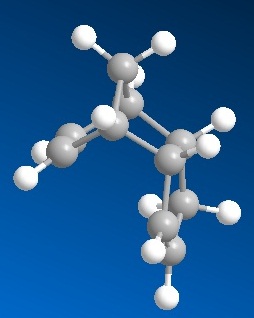
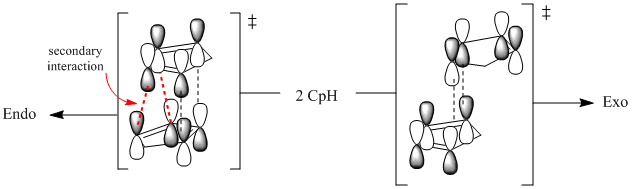
We can see that that total energy of Exo product is 8.9 kJ/mol lower than Endo and the greatest contribution to that is torsional strain in Endo molecule (7.77 kJ/mol higher than in Exo). Therefore we see that in this case Endo is thermodynamic product (lower in energy), although it is not formed. This leads to a conclusion that the reaction is under kinetic control. Since Endo is a kinetic product it means that its transition state is lower in energy and is therefore more stabilized. If reaction is carried out without suplying the system with enough energy to overcome activation barrier for Exo (thermodynamic product, TS higher in energy), only kinetic product is formed. Moreover, when it is already formed, energy barrier for reverse reaction to regenerate cyclopentadiene is even higher, thus kinetic product is trapped. Lower transition state energy for endo product results due to secondary orbital (not HOMO/LUMO) overlap:
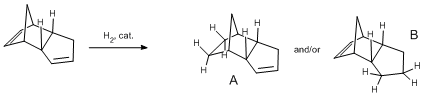
It has been decided to predict the outcome of the hydrogenation of Endo cycloaddition product. The latetr has two double bonds available therefore two different monohydrogenation products can be formed:
Using Molecular mechanics modelling MM2 code both structures were simulated to determine which one is more energetically stable. As previously results are presented in a table below:
| Stretch | Bend | Stretch-Bend | Torsion | non 1-4 VDW | 1-4 VDW | Dipole-Dipole | Total energy | |
|---|---|---|---|---|---|---|---|---|
| A | 4.590 | 60.77 | -2.297 | 52.29 | -4.475 | 18.87 | 0.5883 | 130.3 |
| B | 5.349 | 83.11 | -3.491 | 45.22 | -5.121 | 23.56 | 0.6782 | 149.3 |
Results show that there is much greater bending energy in molecule B that results due to non ideal bond angles around an atom. We can also tell that molecule A has noticeably larger torsional strain energy, which is due to small dihedral anles (basically means some bonds within A are almost eclipsed. Finally molecule A has greater favourable 1-4 Van der Waals energy contribution, which is a result of nuclei attracting each other when they are 2.1< x <2.4 angstroms apart in the molecule. The most pronounced effects (most deviating bond angles, atoms within Van der Waals radii...) of those cases are illustrated in the corresponding molecule models below:
| Most non ideal bond angles in B resulting in high bending energy | torsional strain in A due to small dihedral angles | favourable 1-4 VdW in A | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
From the MM2 optimization results we can tell that product A is thermodynamically favourable, since its overall energy is 19 kJ/mol lower than isomer B. The former has noticeably lower bending and 1-4 Van der Waals interaction energies. Therefore if reaction is carried out at thermodynamic controll we should expect A to predominate, although B might still be a kinetic product aster all. Therefore reaction conditions are important for product controll.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
This section illustrates the application of molecular mechanics to analyse conformations of one of the intermediates in Paclitaxel (sold under trademark TAXOL) synthesis. Carbonyl grou[p in this molecule can exist in two different conformations: either on the same or opposite side of dimethylmethylene bridge on the cyclodecene/cyclohexane fused ring:
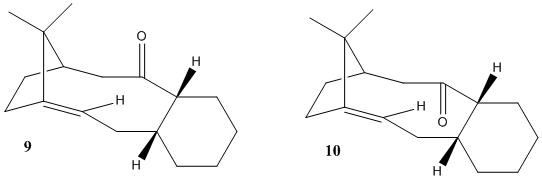
MM2 was used to optimize different conformers of both 9 and 10 to determine the most thermodynamically stable one.
Conformers of 9
This isomer has its carbonyl group on the same side as both marked hydrogens as well as dimethylmethylene bridge. In general it was found to have relatively large total energies reaching up to 560.45 kJ/mol using ChemBio 3D MM2 code (just a number for referrence to compare isomers generated by the same MM2 code). There were two more lower energy conformers found that are shown below:
| Structure | Conformer 9a | Conformer 9b | Conformer 9c |
| Energy (kJ/mol) | 560.45 | 262.76 | 244.30 |
It was found that optimal conformation of five-membered ring part is so called envelope shape in all structures genertated, while cyclohexane part can exist in chair conformation (9a and 9c) as well as in twisted boat (9b). Carbon atom chains joining these two rings then have not that much choice for different conformations, since certain criteria are specified: geometry around the double bond is more or less planar, alkene hydrogen has to be on the same side as methylene bridge, both carbon chains have to be on the same side of cyclohexane ring as well as cyclopentane one. Therefore cyclohexane conformation plays a key role being the most stable in chair conformation.
MMFF94 code was run on all 9a, 9b and 9c conformers to check for any changes and only two structures were isolated (9a and 9c were optimised to thrsame one.):
| Structure | Conformer 9a | Conformer 9b |
| Energy (kJ/mol) | 370.16 | 346.48 |
Since we can not compare the absolute energy values of structures optimised using different codes, I decided to check the energy difference of those two most stable structures optimised by MM2 and MMFF94: 262.76-244.30=18.46 kJ/mol (MM2 case); 370.16-346.48=23.68 kJ/mol (MMFF94 case).
Conformers of 10
Carbonyl group pointing down was found to be more energetically stable and also produced more of low energy conformers that are shown below:
| Structure | Conformer 10a | Conformer 10b | Conformer 10c | Conformer 10d | Conformer 10e |
| Energy (kJ/mol) | 224.81 | 207.15 | 201.71 | 194.26 | 178.57 |
two most stable were further refined with MMFF94 code:
| Structure | Conformer 10d | Conformer 10e |
| Energy (kJ/mol) | 253.34 | 277.98 |
The corresponding energy differences were 194.26-178.57 = 15.69 kJ/mol (MM2), 277.98 - 253.34= 24.64 kJ/mol (MMFF94).
Bridgehead alkenes
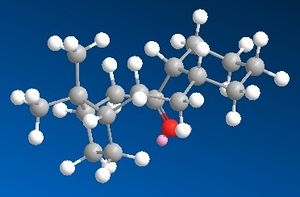
This taxol precursor also belongs to the group of so called Bridgehead alkenes, which have their double bond incorporated into the bridge of bicyclic ring system. Although they may look exotic, these alkenes turn out to be less strained than the corresponding alkanes. The speciffic term is introduced Olefinic strain (OS) to account for how much more stable is alkene than the alkane, which is the difference between their total energies. In our case it is easy to calculate OS of Taxol precursor from MM2 calculations. We only need to optimize the geometry of a corresponding saturated bicycle and take a difference in energies.
In MM2 it was found that saturated version has total energy of 214.89 kJ/mol, while in MMPP94 it was 298.99 kJ/mol, thus SO value can be calculated now in both cases:
SOMM2(taxol prec.)= 214.89 - 178.57 = 36.32 kJ/mol
SOMM2(taxol prec.)= 298.99 - 253.34 = 36.65 kJ/mol
These two are matching each other and confirm that bridgehead alkenes are lower in energy than the corresponding alkanes. It is also the case why they are so unreactive - hyperstable. First, the product is thermodynamically unfavoured and second, its reactivity is reduced due to electronic reasons: ideall flat C=C geometry is twisted in these bicyclic systems and therefore π overlap becomes reduced. This leads to HOMO rise in energy and consequently LUMO lowering (effectively increased HOMO-LUMO separation therefore decreased reactivity).
Modelling Using Semi-empirical Molecular Orbital Theory
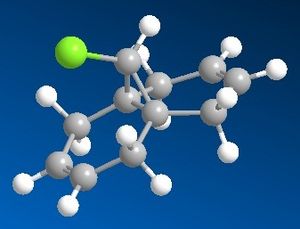
Although molecular mechanics uses far less computing resources it does not take into consideration any quantum mechanical effects in molecules, in other words it knows nothing about its electronic structure, therefore it can not predict some the fact that some molecular orbitals that are close in space within a molecule interact resulting in the change of reactivity and conformation. A good example illustrating this case is a bicyclic chlorinated diene shown in figure 3. This compound is known to be reactive towards electrophiles and frontier orbital calculations therefore would predict which of the two alkenes is more nucleophilic. Moreover C=Cstretching frequency calculations also could tell about electron density (since it effects stretching frequency/bond order). For the latter Gaussian will be used.
Frontier orbitals
For both diene and its hydrogenated version MOPAC/PM6 code was run to predict the shapes of frontier orbitals. Results for chlorinated bicyclic diene molecule are presented below:
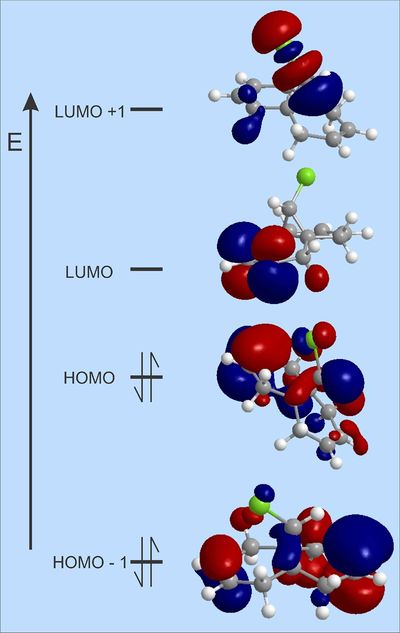
We are interested in HOMO MO, since the latter is responsible towards the reactivity with electrophiles such as dichloro carbene. As we can see its highest coefficient (largest MO lobes) is located on C=C that is endo to chlorine. We can also notice the oposite for HOMO-1 molecular orbital where highest coefficient is on the other C=C, this means that Exo C=C is deeper in energy and more stabilised than Endo. Therefore the latter (Endo) would probably react first under electrophilic attack. It is also worth discussing LUMO of this molecule, which seems to be C=C π* exo to chlorine, which is what we can expect (empty C=C π* are electrophilic) to react with nucleophiles. Similarly LUMO+1 looks like C-Cl δ* that is typically reacting in Sn1 or Sn2 fashion. Moreover if ordering of these MO's is correct, model predicts that nucleophiles will add to C=C (Exo to Cl) rather than replace chlorine on methylene bridge.

Now let's take a look at frontier orbitals of hydrogenated version of the same molecule. HOMO remains in the same position and shape, still with the largest coefficiens on C=C π bonding fragment. HOMO-1, on the other hand, looks completely different, since the other alkene does no longer exist (that is where HOMO-1 was located before). It now looks like C-Cl π* MO fragment. LUMO and LUMO+1 look very much the same as before but now they swapped places - C-Cl should react easier with nucleophiles than C=C.
Vibrational frequencies
To evaluate the effect of C=C on C-Cl stretching frequency, Gaussian DFT method (B3LYP/6-31G(d,p)) was run to optimise the geometry (after initially optimising with MM2 and MOPAC/PM6) and to predict IR stretching frequencies. Results are presented below:
| Molecule | ν (C=C endo to chlorine)
/ cm-1 |
ν (C=C exo to chlorine)
/ cm-1 |
ν (C-Cl)
/ cm-1 |
|---|---|---|---|
| Bridged chlorodiene http://hdl.handle.net/10042/to-10341 | 1757 | 1737 | 771 |
| Hydrogenated bridged chlorodiene http://hdl.handle.net/10042/to-10340 | 1754 | --- | 780 |
Since we know that stronger bonds give higher stretching frequencies (due to higher force constant in SHO model), it becomes evident that C-Cl bond gets weakened in the presence of C=C exo to it. C-Cl frequency becomes 9 cm-1 higher when C=C is hydrogenated. One explanation is that electron density from electron rich C=C π MO is donated to C-Cl σ* MO, which weakens it (decreases its ν).
Monosaccharide chemistry: glycosidation
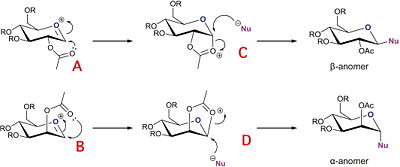
In this part I am going to examine the energies and thus relative stabilities of A and A' that carry an acyl group on the carbon next to oxonium ion in six membered monosaccharide ring (see figure 6).
First MM2 simulations were run to obtain energies and structures of pairs A&A' and B&B'. The most stable are presented below:
| Conformer | A | A' | B' | B |
| Energy / (kcal/mol) | 116.90 | 128.70 | 98.12 | 86.61 |
Although MM2 code allows both pairs of conformers, which resemble how molecules twist and rotate around single bonds to be able to react intramolecularly, which leads to products C and D. On the other hand PM6 protocol takes orbital interaction into account and therefore spots acetyl oxygen's lone pair electrons talking to C=O π* in oxonium ion, which if they were to overlap would lead to ring closure and overall energy lowering of the molecule. This is a favourable intetraction, thus it is hard to get X' conformers, that are not set up for this ring closure reactions. Structures from PM6 optimisation are presented below:
| Conformer |
|
----- |
|
| |||||||||
| Heat of formation / (kcal/mol) | -383.5 | ---- | -320.29 | -342.33 |
When comparing the structures generated with MM2 ang PM6, side chains in these will not be discussed in details
Eventually acyl oxygen attacks cyclic oxonium ion to form another 5-membered rings C and D respectively. MM2 simulations of those give the following lowest energy conformers:
| Structure | C | D | ||||||
|---|---|---|---|---|---|---|---|---|
| Model |
|
| ||||||
| Energy (kJmol) | 143.26 | 192.21 |
| Structure | C | D | ||||||
|---|---|---|---|---|---|---|---|---|
| Model |
|
| ||||||
| Energy (kJmol) | 383.51 | 351.79 |
For molecules C and , PM6 gives the same C and O separation before and after closure of 5-membered ring, which means it already approximated the existance of bond in open cycle structure. Moreover, the energies of them are identical, thus further confirming this hypothesis. However this does not allow to calculate stabilisation energy due to neighboring group participation. For the pair B/D energy difference however exists and it is 9.46 kJ/mol favouring cyclic intermediate. MM2 energy calculations favour non cyclic version, most likely due to the fact that molewcular mechanics take into account extra bending and torsional energy upon closing the ring rather than interacting orbitals that would lower molecule's energy upon overlap.
Mini project: Enantioselective reduction of Tetrabenazine
Dihydrotetrabenazine (DTBZ) is involved in vesicular monoamine transport and thus important in dopamine and other neurotransmitter migration into synaptic cleft. It has been found to be a metabolite of clinically administrated drug Tetrabenazine (TBZ, that is sold under trademark Nitoman), which functions as VMAT2 inhibitor and is used mainly to treat Huntington's disease, Hemiballismus and other neurological diseases.
Young Wook Son and coworkers found that ketone in Tetrabenazine molecule can be selectively reduced to the corresponding alcohol predominantly forming only one diastereomer:
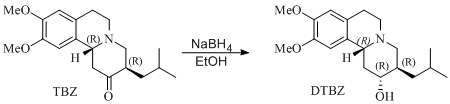
Researchers identified reduction product to be DTBZ by comparing their spectroscopic data to literature results where absolute stereochemistry is known. Although in my miniproject an alternative approach is utilised. The idea is to predict 13C NMR of both DTBZ diastereomers (where stereochemistry is inverted at carbon bearing hydroxy substituent) and compare with that obtained by Young Wook Son et.al. to account for the reaction outcome.
First conformation of both versions of DTBZ and starting material TBZ were optimised using MM2 code. There were a couple of low energy ones for both alcohols, which I settled to, yet upon optimising TBZ carbonyl group carrying cyclohexane ring was forced into nearly perfect chair, which made me reconsider previous alcohol conformers and helped to bring them around 4 kcal/mol down in energy and tweaking butyl chain to point away from carbonyl gave the intermediate structures. They were further refined using PM6 code and finally optimized using Gaussian DFT/MPW1PW91/6-31G(d,p) protocol to yield the following structures:
| Molecule | DTBZ (1) | DTBZ (2) | TBZ precursor |
| Authenticity | http://hdl.handle.net/10042/to-10334 | http://hdl.handle.net/10042/to-10335 | http://hdl.handle.net/10042/to-10337 |
Supporting notes of the original Young et.al. paper gives these values for the TBZ and DTBZ NMR:
Tetrabenazine 13C NMR (100 MHZ, CD3OD) δC ppm: 21.11, 22.08, 25.20, 28.51, 34.87, 46.14, 46.37, 49.72, 54.94, 55.18, 60.66, 62.20, 108.38, 111.56, 125.97, 128.40, 147.58, 148.07.
Dihydrotatrebenazine 13C NMR (75 MHz, CDCl3): δC ppm: 21.76, 24.15, 25.36, 28.60, 29.72, 39.62, 40.23, 41.22, 51.68, 55.87, 55.97, 59.83, 60.93, 74.30, 107.88, 111.44, 126.03, 128.70, 147.36, 147.69.
Those refined geometries then were used to predict 13C NMR spectra of all three molecules above, which gave the following results:
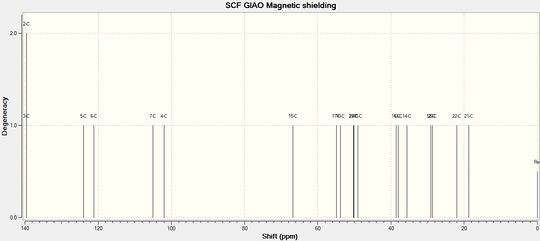
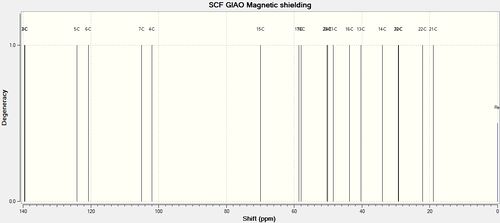
Numerical values of predicted 13 C NMR using TMS mPW1PW91/aug-cc-pvdz CDCl3 GIAO reference are as follows:
Dihydrotetrabenazine (1)and (2) 13C NMR (CDCl3): δC ppm:
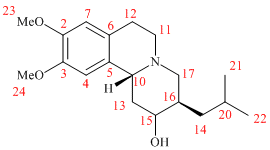
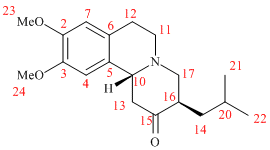
| # Carbon | DTBZ (1) | DTBZ (2) | TBZ |
|---|---|---|---|
| 3-C | 139.56 | 139.54 | 139.43 |
| 2-C | 139.52 | 139.42 | 139.58 |
| 5-C | 123.95 | 124.05 | 123.27 |
| 6-C | 121.16 | 120.69 | 121.27 |
| 7-C | 105.03 | 105.02 | 105.23 |
| 4-C | 101.94 | 102.01 | 101.95 |
| 15-C | 66.82 | 69.95 | 203.38 |
| 17-C | 54.87 | 58.65 | 59.39 |
| 10-C | 53.80 | 58.00 | 59.29 |
| 23-C | 50.24 | 50.32 | 50.37 |
| 24-C | 50.14 | 50.09 | 50.17 |
| 11-C | 49.08 | 48.46 | 47.90 |
| 16-C | 38.52 | 43.67 | 48.73 |
| 13-C | 38.04 | 40.28 | 45.46 |
| 14-C | 35.57 | 33.99 | 29.10 |
| 12-C | 29.08 | 29.23 | 29.18 |
| 20-C | 28.59 | 29.31 | 28.39 |
| 22-C | 22.03 | 22.14 | 21.80 |
| 21-C | 18.70 | 18.95 | 18.91 |
Discussion
We are interested in the shift corresponding to C-15, since it changes very noticeably during the reaction. In TBZ C-15 is doubly bonded to oxygen and predicted peak is at 203.38 ppm (207.44 if corrected), while litrature[2] gives it to be 210 ppm. Although in product after reduction this peak disappears and a new one appears at 74.30 ppm, which must be carbon 15. The predictions for both diastereoisomers are 69.95 (DTBZ-2) and 66.82 (DTBZ-1). Although these results are not quite a good match, predicted peak for DTBZ(2) is closer to the one observed, and indeed it is the same molecule authors claim to have synthesized.
Another unambiguous way to assign the geometry at hydroxy in the product is to predict proton's (attached to C-15) coupling constants and compare them with literature values. To do so, Gaussian optimised DTBZ(2) structure was submitted for spin-spin coupling constant prediction using Gaussian DOI:10042/to-10488 to give the following results:
 9.5(JAB); 4.3(JAC); 2.0 (JAD). However the peak of this proton appears as a multiplet at
9.5(JAB); 4.3(JAC); 2.0 (JAD). However the peak of this proton appears as a multiplet at
2.51-2.56 appears as a multiplet and unfortunatelly is impossible to interpret unless higher frequency apparatus is employed to record it.
References
[1] Young Wook Son, Tae Hui Kwon, Jae Kyun Lee, "A Concise Synthesis of Tetrabenazine: An Intramolecular Aza-Prins-Type Cyclization via Oxidative C<H Activation", Org. Lett., DOI:10.1021/ol202792q
[2] Sung-Whi Rhee, Kenneth J. Ryan, and Mary J. Tanga, "Synthesis of 3H-Labeled Tetrabenazine (TBZ)" DOI:10.1002/jlcr.1881
[3]Douglas M. Jewett, Michael R. Kilbourn, Lihsueh C. Lee,"A simple synthesis of [11C]Dihydrotetrabenazine (DTBZ)"DOI:10.1016/S0969-8051%2896%2900213-2