Rep:Mod1:Yuko.Isayama3001
The Basic Techniques of Molecular Mechanics and Semi-Empirical Molecular Orbital Methods for Structural and Spectroscopic Evaluations
Modelling Using Molecular Mechanics
Molecular mechanics uses classical mechanics to model a molecular system; the methods applies Hooke's Law to describe the force field in which atoms move and it involves determining the optimum geometry of a molecule to calculate a steric energy which is analysed in terms of the bond length and angle strain, steric effects and van de Waals contributions. However, the method has its limitations which were recognised during its application to the following chemistry problems.
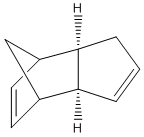
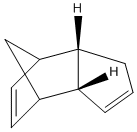
The Hydrogenation of Cyclopentadiene Dimer
 |
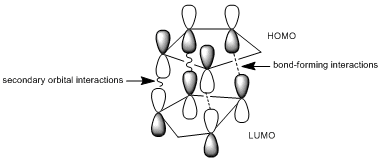 |
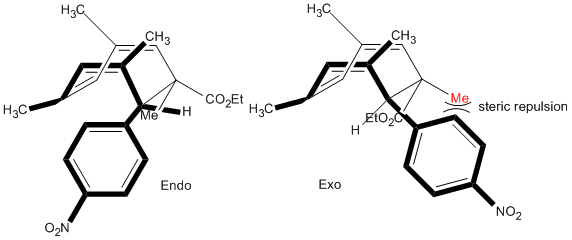
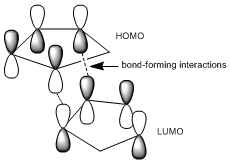
By optimising the geometries of both isomers 1 and 2, their total energies are calculated as 133.42 and 142.31kJ/mol, respectively, and thus, these values suggest that the endo-product is less stable and yet it is preferred in Diels-Alder reactions. The preference for endo-stereochemistry is observed due to secondary orbital interactions between the HOMO of the diene and the LUMO of the dienophile. This interaction does not lead to the formation of new bonds but contributes to the stabilisation of endo-transition state with respect to that of the endo-one, suggesting that it is formed under kinetic control if the Diels-Alder reaction is irreversible. The exo-dimer is more stable thermodynamically because it appears less sterically hindered and so is said to be formed under thermodynamic control.
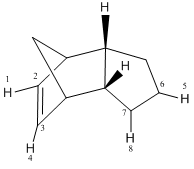
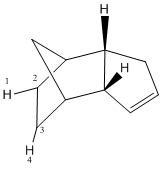
Referring to the table below, the significant differences between the steric energy terms for isomers 3 and 4 include the bending and torsion terms; isomer 3 displays a larger bending term because the H(1)-C(2)-C(3)bond angle is deformed from an optimal value of 120.0° to 126.9° whilst the corresponding H(1)-C(2)-C(3) angle in isomer 4 is 110.4°, much closer to the optimal value of 109.4°. With regards to the torsional strain, 4 has a larger torsion term due to the H(5)-C(6)-C(7)-H(8) dihedral angle of 0.41° compared to a restricted H(1)-C(2)-C(3)-H(4) angle of 0.02°as a result of steric effects in 3. Nevertheless, the bending, stretching and van de Waals terms for isomer 4 are all smaller which explains why 4 is more stable than 3.
Comparison of hydrogenated isomers 3 and 4
| Energy Term | Isomer 3/kJmol-1 | Isomer 4/kJmol-1 |
|---|---|---|
| Bending | 82.87 | 60.72 |
| Stretching | 5.30 | 4.22 |
| Torsion | 45.48 | 52.33 |
| Van der Waals | 23.60 | 18.86 |
Minimised models of 1, 2, 3 and 4 by MM2 field option
 |
 |
 |
 |
References
- J. Clayden, N. Greeves, S. Warren and P. Wothers, Organic Chemistry, Oxford University Press, 2001, 916
- R. O. C. Norman and J. M. Coxon, Principles of Organic Synthesis, 3rd edition, Chapman and Hall, 1993, 273
- F. A. Carey and R. J. Sundbergy, Advanced Organic Chemistry: Structure and Mechanism, 4th edition, Plenum Publishers, 2000, 124
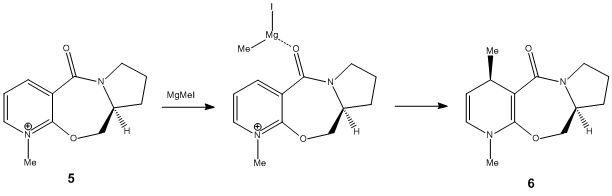
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ analogue)
 |
 |
Conformation minima of 5
|
|
There is very little flexibility in the seven-membered ring limiting to only two conformation minima of 5, which focus on the geometry of the amide carbonyl group and its orienation with respect to the pyridinium ring; one in which the amide carbonyl group is above the plane of the pyridine ring by 129.6°(5.1; 303.87kJ/mol), and another in which the carbonyl group and the pyridinium ring are in the same plane (5.2; 180.38kJ/mol). However, optimisation of 5 by the MM2 method shows that it not possible to have a conformer with the amide carbonyl group positioned below the pyridinium ring.
Stereochemistry of 6 can be rationalised by coordination between the Grignard reagent and the amide oxygen atom as shown in the intermediate step. Since it was concluded that the amide carbonyl group cannot be positioned below the pyridnium ring, product 6 can only exist with the methy group positioned upwards. More so, the simple model can be improved by including the MgMeI componenet to study the transition state of the reaction. However, this poses a problem when applying the MM2 field option since it does not allow calculation of the total energy of the molecule; the metal ion, Mg2+, is unidentified and cannot be easily handled by this method. It is possible to study and optimise the transition structure by referring to Module 3, using the QST2 method, which would be able to further support the observed sterochemistry of 6.
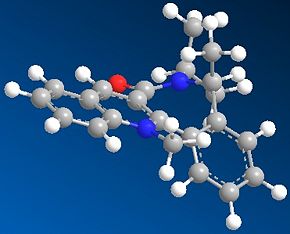
Minimum conformation of 7
|
Similar to the previous example, the rigidity of the fused benzene rings as well as the seven-membered ring presents limited conformations. In fact, the geometry of the amide carbonyl group can only be based below the plane of the aromatic ring, producing conformer 7.1 with a total energy of 261.98kJ/mol.
Stereocontrol in the formation of 8 is due to the O-N lone pair repulsions between the NHPhenyl and carbonyl group. Thus, the position of the carbonyl group will influence the attack of PhNH2 from the top face of the molecule.
References
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
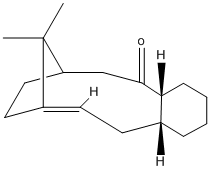
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
 |
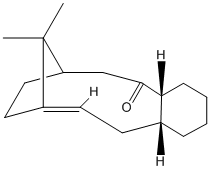 |
A key intermediate 10 or 11 in the total synthesis of Taxol is initially synthesised with the carbonyl group pointing either up or down. By using MM2 method the most stable isomer is determined as 11 with a total energy of 188.13kJ/mol, whilst isomer 10 is 204.71kJ/mol. A particular aspect of the structure that was improved by further minimisation was to adjust the conformation of the six-membered ring so that it adopts a chair rather than boat conformation as well as further adjusting the dihedral angle of the carbonyl group with respect to the six-membered ring for each isomer.
MM2 minimisation allows to find the lowest energy of conformation of a molecule, but, it must be noted that this does not necessarily find the global minimum, but may locate a local minimum in the energy. Thus, it cannot be known whether my determined energies of either isomers are actually the global minimum. Finding the global minimum will depend on how close the starting structure corresponds to the global minimum's conformation. Therefore, it is necessary to study many trial geometries as a starting point so that a global minimum for a given molecule can be identified.
Trial geomteries of 10
| Steric energy/kJmol-1 | Dihedral angle of carbonyl w.r.t six-membered ring/° |
|---|---|
| 357.0 | 36.7 |
| 249.8 | 53.4 |
| 227.0 | 55.5 |
| 226.6 | 56.0 |
| 204.7 | 53.4 |
Trial geomteries of 11
| Steric energy/kJmol-1 | Dihedral angle of carbonyl w.r.t six-membered ring/° |
|---|---|
| 420.6 | 131.4 |
| 214.5 | 117.8 |
| 212.7 | 176.5 |
| 201.6 | 171.1 |
| 188.13 | 177.1 |
|
|
Olefin strain energy (OSE) is defined as the difference between the strain energy of an olefin and the corresponding saturated hydrocarbon. Generally, the olefin is more strained than the alkane, but for some systems the olefin is actually less strained than the parent carbon with negative olefin strain energies and this is because of the bridgehead location of the double bonds which are present in isomers 10 and 11; these class of compounds are known as 'hyperstable alkenes' and are characterised by their reduced reactivity during subsequent fucntionalisation.
References
- Maier, W. F.; Schleyer, P. V. R, J. Am. Chem. Soc. 1981, 103, 1891. DOI:10.1021/ja00398a003
Modelling Using Semi-Empirical Molecular Orbital Theory
Molecular mechanics approach has its limits since it does not take into account the secondary orbital interactions to explain the endo-selectivity in Diels Alder cycloadditions. An alternative approach in which the electronic aspects of reactivity can be illustrated is the semi-empirical molecular orbital theory; such calculations consider probability of electron distribution in the molecule, and how they influence bonds and explain the spectroscopic properties.
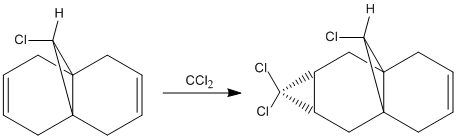
Regioselective Addition of Dichlorocarbene
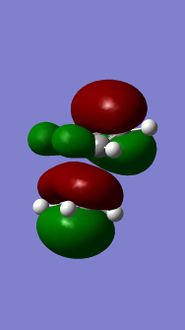
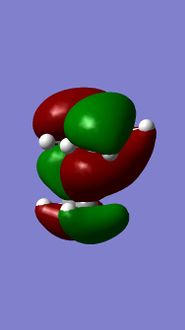
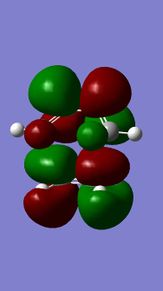
Molecular orbitals of 12
 |
 |
 |
 |
 |
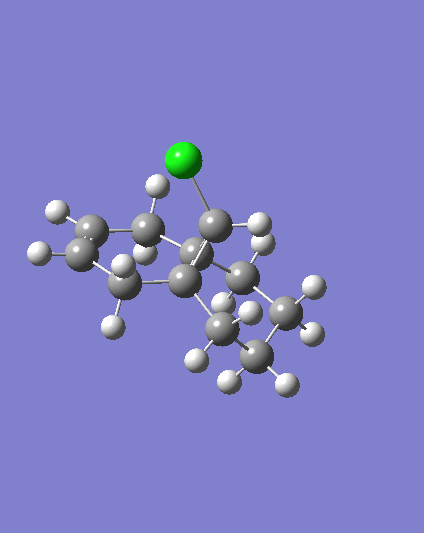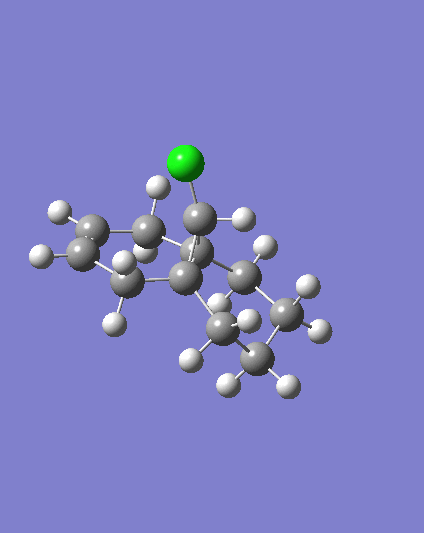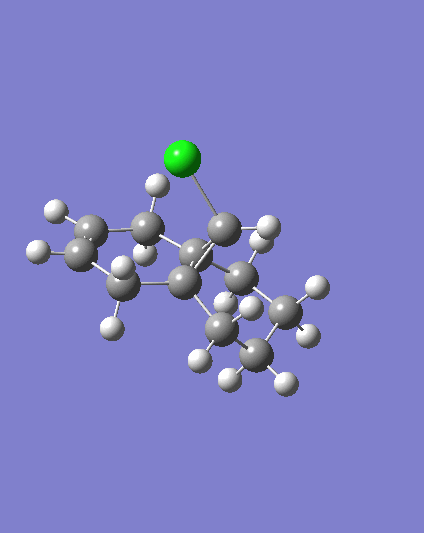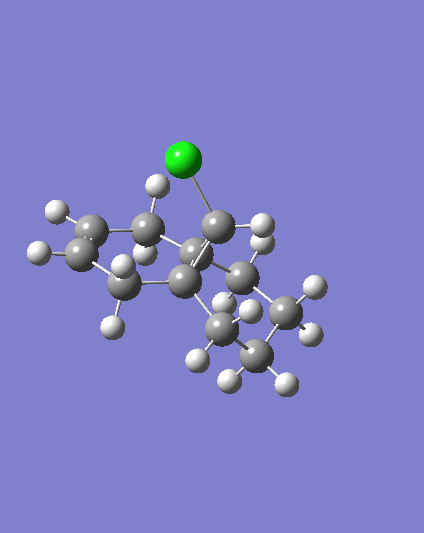
MM2 minimzation of 12 produced a conformation in which the two rings are geometrically distorted significantly, with the distance between either of the exo-double bond carbons and the central bridgehead carbon being shorter than the corresponding endo-distance. This is a result of an antiperiplanar stabilisation effect induced by the Cl-C o* (LUMO+1) orital interacting with the exo-π (HOMO-1) orbital; the occupied π-orbital donates electron density into the antibonding Cl-C orbital, thus weakening the C-Cl bond but overall this effectively lowers the energy of the molecule. The fact that electron density is being donated from the exo-π orbitals explains the observed selectivity of the endo-double bond by electrophiles.
Two molecules were compared; compound 12 which contains a double bond anti to the Cl-C bond and a hydrogenated version where this anti (or exo) double bond is replaced by a C-C single bond, in order to calculate the influenece of the Cl-C bond on the vibrational frequencies of the molecule. Specifically, the two C=C stretches for the diene and the single C=C stretch for the monohydrogented derivative were also identified;
| Bond | Compound 12/cm-1 | Compound 12.2/cm-1 |
|---|---|---|
| C-Cl | 772.6 | 776.9 |
| C=C (endo to Cl) | 1761.0 | 1761.7 |
| C=C (anti to Cl) | 1740.28 | n/a |
Hydrogenating the exo-double bond leads to the absence of an exo-π orbital so that there is no longer a C-Cl o* exo-π orbital interaction where electron density is donated into the C-Cl o* orbital. Consequently, the C-Cl bond in 12.2. appears as a stronger bond which accounts for the higher vibrational frequency.
 |
 |
References
- B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- B. Halton and S. G. G. Russel, J. Org. Chem., 1991, 56. DOI:10.1021/jo00019a015
Structure Based Mini Project Using DFT-based Molecular Orbital Methods
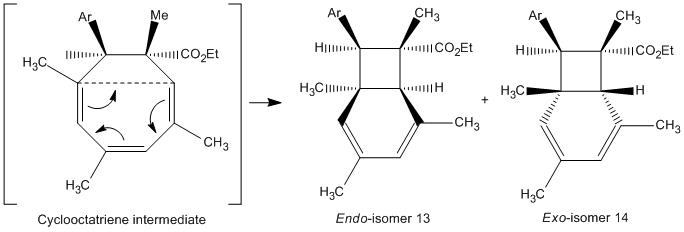
A reaction which gave two isomeric products of endo- and exo-bicyclo[4.2,0]-octadiene isomers was studied.
|
By forming crystals of each isomeric product, x-ray crystallography can be used to determine arrangement of atoms within the crystal and the key spectroscopic difference would be the orientation of the six-membered ring of whether it is above or below the plane of the 4-membered ring to give either the endo- and exo- confomation. It would not be possible to distinguish between the two isomers in terms of dihedral angles which relate to the 3JH-H</sub, > coupling constants using the Karplus equation; with focus on the orientation of the six-membered ring, there is no three-bond coupling in the rings of each isomer. Thus, whilst 1H NMR data is not sufficient, confirmation of stereochemistry can be determined by NOESY technique which relies on through-space proton interactions instead of J-couplings.
The predicted 13C NMR and IR specta were analysed for each isomer using the GIAO method producing the following data;
Endo-isomer
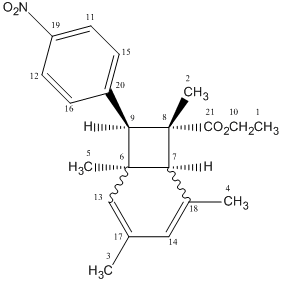 |
13C NMR (CDCl3)
| Carbon label | Predicted δ/ppm | Experimental δ/ppm | ppm difference |
|---|---|---|---|
| 1 | 15.5 | 14.3 | 1.2 |
| 2 | 16.3 | 14.7 | 1.6 |
| 3 | 23.6 | 22.5 | 1.1 |
| 4 | 24.0 | n/a | n/a |
| 5 | 31.9 | 29.9 | 2.0 |
| 6 | 44.4 | n/a | n/a |
| 7 | 51.1 | 49.1 | 2.0 |
| 8 | 51.4 | 50.4 | 1.0 |
| 9 | 57.4 | 57.7 | 0.3 |
| 10 | 60.6 | 61.0 | 0.4 |
| 11 | 120.4 | n/a | n/a |
| 12 | 121.3 | n/a | n/a |
| 13 | 122.7 | 122.8 | 0.1 |
| 14 | 123.1 | 123.1 | 0.0 |
| 15 | 126.7 | 124.7 | 2.0 |
| 16 | 130.1 | 130.3 | 0.2 |
| 17 | 130.3 | 130.7 | 0.4 |
| 18 | 131.9 | 131.6 | 0.3 |
| 19 | 142.9 | 145.3 | 2.4 |
| 20 | 144.5 | 146.8 | 2.3 |
| 21 | 177.1 | 177.2 | 0.1 |
IR
| Bond | Predicted /cm-1 | Experimental /cm-1 | % difference |
|---|---|---|---|
| C-H stretch (aromatic ring) | 3025 | 2973 | 1.7 |
| C=O stretch | 1795 | 1721 | 4.3 |
| C=C stretch (aromatic ring) | 1535 | 1517 | 1.2 |
| N=O stretch | 1397 | 1346 | 3.8 |
Exo-isomer
13C NMR (CDCl3)
| Carbon label | Predicted δ/ppm | Experimental δ/ppm | ppm difference |
|---|---|---|---|
| 1 | 15.3 | 14.3 | 1.0 |
| 2 | 23.2 | 22.0 | 1.2 |
| 3 | 23.8 | 22.5 | 1.3 |
| 4 | 25.3 | 25.1 | 0.2 |
| 5 | 28.0 | n/a | n/a |
| 6 | 41.9 | 39.2 | 2.7 |
| 7 | 54.9 | 55.1 | 0.2 |
| 8 | 57.5 | 56.2 | 1.3 |
| 9 | 61.0 | 61.1 | 0.0 |
| 10 | 61.9 | n/a | n/a |
| 11 | 120.7 | n/a | n/a |
| 12 | 121.5 | n/a | n/a |
| 13 | 122.1 | 123.2 | 1.1 |
| 14 | 124.7 | 123.5 | 1.2 |
| 15 | 125.8 | 126.4 | 0.6 |
| 16 | 128.0 | 127.9 | 0.1 |
| 17 | 130.7 | 131.2 | 0.5 |
| 18 | 131.8 | 132.2 | 0.4 |
| 19 | 142.8 | n/a | n/a |
| 20 | 143.6 | 146.7 | 3.1 |
| 21 | 175.5 | 175.2 | 0.3 |
IR
| Bond | Predicted /cm-1 | Experimental /cm-1 | % difference |
|---|---|---|---|
| C-H stretch (aromatic ring) | 3025 | 2960 | 2.2 |
| C=O stretch | 1788 | 1714 | 4.3 |
| C=C stretch (aromatic ring) | 1535 | 1515 | 1.3 |
| N=O stretch | 1397 | 1344 | 3.9 |
The predicted 13C NMR data are in relatively good agreement with the experimental data with the largest difference of 2.7ppm which suggests that computational technique is accurate enough to predict the correct chemical shifts of this particular compound. Absence of some of the experimental couplings is likely to be due to rapid rotations that the C atoms undergo so that they all experience the same chemical environment, leading them to show one signal in the 13C NMR. Whilst, computational technqiues analyses a static molecule in which every chemical shift of each C atom is assigned. Looking at the data, it can be seen that both isomers have similar coupling constants so this method may not be reliable enough method to distinguish between them and further analysis using other spectroscopic techniques mentioned above may need to be carried out.
Comparison of the experimental IR data with that of the predicted data shows that again, they are in good agreement. However, there is not enough IR data to draw relevants conclusion concerning the accuracy of the computational method; the literature only quotes the significant vibrational frequencies.
Key literature
- K. A. Parker and Y. H. Lim, Organic Letters, 2004, 6, DOI:10.1021/ol036048+
- D. L. Pavia, G. M. Lampman and G. S. Kriz, Introduction to Spectroscopy, 4th edition, Brooks/Cole, 2009