Rep:MgOHJK114
Introduction
Atoms can be vibrating in the order of 10% of the interatomic distance.[1] Therefore an understanding of crystal vibrations is needed in order to fully characterise materials. Important applications of this understanding include:
- how sound waves propagate through materials
- how light interacts with matter
- calculation of thermodynamic properties
- thermal expansion
- thermal conductivity
- phase transitions
- superconductivity
Why do we do Molecular Dynamics/Lattice Dynamics? To model systems that cannot be modelled experimentally and to calculate thermodynamic properties In this lab the thermal expansion of the simple ionic crystal, MgO, is investigated.
Three-dimensional crystal lattices can be classified into 14 different general types; known as Bravais lattices. A Bravais lattice is an infinite array of discrete points that is identical in appearance from whichever of the points the array is viewed; it displays translational invariance. Consequently, any observable property of the lattice can be described by Bloch functions where Bloch’s theorem relies on this property of translational invariance. Bloch’s theorem leads to the Fourier transformation of a ‘real’ space basis set to a reciprocal space set of eigenstates that are labelled with specific k values. K is known as the wave vector and, in the context of crystal vibrations, it labels each vibration in reciprocal space; also known as k-space or momentum space since p=ћk. However, this is not the classical momentum since the Heisenberg uncertainty principle states that it is not possible to know both the position and momentum of a particle. Therefore, at absolute zero the atoms in a crystal must vibrate about their equilibrium position and this vibrational energy is known as the zero point energy.
The amplitude of these vibrations increases with temperature as the atoms gain more thermal energy. When the atoms vibrate with a small amplitude they can be treated as independent, simple harmonic oscillators and this small amplitude limit is known as the harmonic limit. Anharmonic effects, such as thermal expansion, arise when the amplitude of vibration is large e.g. around the melting point of the solid. The harmonic approximation ignores the higher-order terms, known as anharmonic terms, in the Taylor series expansion of the potential energy. In the harmonic approximation the frequency of the lattice mode is independent of volume.
Calculating the Internal Energy of an MgO Crystal
Lattice Vibrations- Computing the Phonon Modes
The gradient of the vibrational band structure (phonon dispersion) represents the degree of repulsion between the vibrating atoms. In metals the vibrating metal cations are shielded from each other by surrounding, delocalised electrons. Whereas, in simple ionic crystals such as MgO, the ions (with a symmetric closed-shell charge distribution) are not shielded from each other and so the gradient is large and variable.
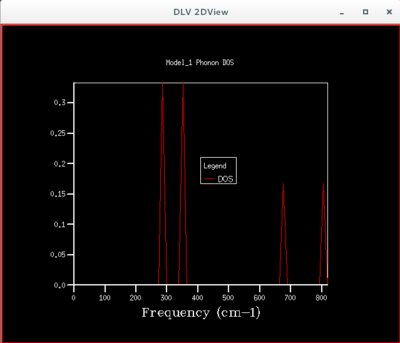
The density of states is a useful object which summarises the dispersion curves - it is an average over all k-points yielding the number of vibrational modes at each frequency (the density of modes).

This single k point is k point L since the frequencies from the dispersion curve in fig.1 correspond to the frequencies in fig.2. As the grid size increases more and more of the possible vibrations are sampled and more features appear. How does the density of states computed at this optimal grid size compare to that computed for smaller and larger grids ? How is the density of states related to the dispersion curves ?

Lattice Dynamics
Lattice Dynamics (LD) is the study of vibrations in crystals. The key approximation used in LD is the harmonic approximation. This is effectively the only approximation that has an exact solution. When the potential energy between 2 atoms is expanded in a Taylor series around the minimum, equilibrium distance between them, it looks like this:
TAYLOR EXPANSION THING
The harmonic approximation ignores the higher-order terms, known as anharmonic terms, in the Taylor series expansion of the potential energy. These anharmonic terms give rise to effects such as thermal expansion, thermal conductivity and the behaviour of a system around phase transitions.[1]
Molecular Dynamics
Molecular Dynamics (MD) uses Newton's Second Law to classically solve the motion of Lagrangian particles (atoms, molecules). Its value lies in the simplicity of its mathematical formulation, which leads to efficient calculation of the thermodynamic properties of a system which evolve and update with each timestep of the calculation[2]. The force acting on each atom of the system can be obtained by the summation of the derivative of the interatomic potential energy surface. If the interatomic potential is known, then the force acting on the atoms can be simulated and so the evolution of the system's behaviour can be determined.
- is the force acting on atom i.
- is the mass of atom i.
- is the acceleration of atom i, the rate of change of its velocity.
- is the velocity of atom i.
- is the position of atom i.
Melting Point of MgO
The melting point of MgO is around 3250 K[3]
