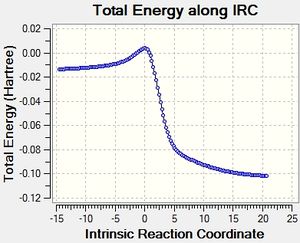
Rep:Mea15TS
Introduction
In the course of this lab 3 different systems that mainly undergo Diels-Alder reactions were analysed. These reactions were analysed using the Gaussian software.
The Gaussian software generates a potential energy surface with 3N-6 dimensions, where N is the number of atoms analysed, from the structure of the molecule inputted, this potential energy surface is then probed to find important points such as minima and transition states of the molecule which can them be used to predict chemical reactivity and the mechanism of the reaction among other things.
A transition state is defined as a saddle point with only one negative force constant present on a potential energy surface. A minima on a potential energy surface has no negative force constants. The force constants are obtained by finding the second derivative of the PES (curvature). A minimum point will have a gradient of zero and a positive curvature for all of the derivatives while for a transition state the gradient is zero for all the derivatives but the curvature is positive for all of the derivatives bar one which will have a negative curvature, this explains why all transition state have one negative frequency associated with them and all minima just have positive frequencies
Two different methods of computing in Gaussian were used for these experiments, PM6 which is a semi empirical method that involves a Hartree-Fock calculation[1] but some of the more complex terms are approximated based on experimental data or excluded[2]. This makes these calculations fast but unreliable in some instances due to the approximations used. Meanwhile B3LYP/6-31G(d) is a DFT (density functional theory) method with a basis set including s and p +d orbitals. This is a more accurate method[3] due to the Hartree-Fock calculation being solved and in addition factors such as exchange correlation being added, in this method B3YLP stands foe the Hartree Fock calculation being solved and exchange being included while the 6-31G(d) refers to the basis set used.
The Diels-Alder systems were analysed using the computational methods explained above and the information obtained was used to analysed various properties of these reactions to find if the results matched theory.
Nf710 (talk) 17:34, 17 November 2017 (UTC) This is a nice intro. B3LYP is a DFT method that solve the DFT hamiltonian but accounts for the exchange correlation with a Hatree fock calculation and is therefor known as a hybrid functional.
Exercise 1: Reaction of Butadiene with Ethene
Reaction overview

A Diels-Alder reaction is a [4+2] cycloaddition reaction between a diene and a dienophile as shown by the mechanism.
Below the transition state of this simple Diels-Alder reaction is analysed and a frontier MO diagram for this reaction is drawn, this diagram is then used to explain rules which the reaction must obey. The actual MO's of the reaction are also visualised and associated with the MO diagram.
In addition bonds lengths are analysed over the course of the reaction and compared to standard values and important bond forming vibrations are visualised and analysed.
For this analysis the products, reactants and transition state were optimised to the PM6 level to visualise the MO's and vibrations. In addition an IRC was taken to record how the bonding changed over the reaction.
MO diagram and analysis of MO's
MO diagram
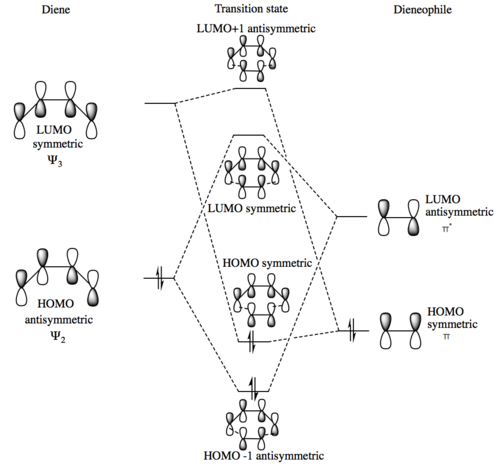
The MO diagram shows the HOMO of the diene interacting with the LUMO of the dienophile strongly while the HOMO of the dienophile reacts with the LUMO of the diene weakly. This forms 4 transition state molecular orbitals of varying energies. This simple Diels-Alder follows normal electron demand. Below are images of all of the orbitals present on the MO diagram, the names of the orbitals associate them to the relevant orbitals on the MO diagram above.
(Fv611 (talk) Even though you know that only S/S and AS/AS fragment orbitals can combine to form an orbital of the same symmetry, you still indicated the TS LUMO to be a symmetric combination of two asymmetric orbitals, and the TS LUMO+1 to be an antisymmetric combination of two symmetric orbitals. A shame because everything else in this exercise is correct.)
Butadiene MO's
|
|
|---|
Ethene MO's
|
|
|---|
Transition state MO's
|
|
|
|
|---|
Analysis of MO's
The interactions of the orbitals show that reactions can only occur if each the HOMO and LUMO present in each HOMO-LUMO interaction shares the same symmetry. eg an antisymmetric HOMO interacts with an antisymmetric LUMO. A HOMO-LUMO interaction that is antisymmetric-symmetric will therefore not occur and hence the reaction is forbidden. This is shown in the frontier MO diagram and in the real transition state MO's that have been visualised above.
Antisymmetric-antisymmetric and symmetric-symmetric interactions have a non zero overlap integral as the orbitals will either overlap all in phase or all out of phase depending on if the TS MO produced is bonding or anti-bonding. Meanwhile a symmetric-antisymmetric interaction will have a zero overlap integral as in case above one pair of orbitals will overlap in phase and one set will overlap out of phase, this will produce a net overlap across the molecule of nothing as the stabilisation gained from the in phase overlap would be cancelled out by the destabilisation of the out of phase overlap thus leaving the entire overlap integral to be 0.
Bond lengths over the course of the reaction and bond forming vibrations
Bond lengths
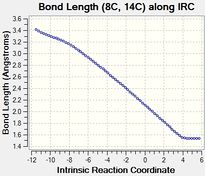
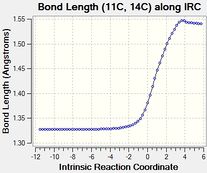
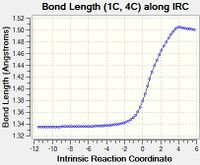
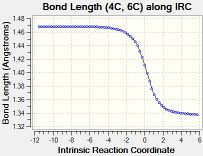
The table below tabulates how the C-C bond lengths of the reactants changed over the course of the reaction:
The C-C bond lengths of the reactants are 1.34 angstroms for a double bond and 1.47 angstroms for a single bond in butadiene. In ethene the double bond is 1.33 angstroms.
The C-C bond lengths of the transition state are 1.38 for the 3 double to single bonds, 1.41 angstroms for the double to single bond and 2.11 angstroms for the bonds being formed.
The C-C bond lengths of the products are 1.50 and 1.53 angstroms for the single bonds and 1.34 angstroms for the double bond
The bond lengths change by 0.16-0.19 angstroms for a double to single bond and 0.13 angstroms for a single to double bond over the course of the reaction. The single bonds originally are closer in character to the final double bonds than the double bonds being closer in character to the final single bond due to the original single bond being part of the butadiene molecule and hence having some double bond character due to the the conjugation of the double bonds.
The typical sp3 and sp2 C-C bond lengths are 1.54 angstroms and 1.34 angstroms respectively[4]. sp3 bond lengths are shorter than what is expected usually in the reactants due to the conjugation of the double bonds in butadiene which causes the single bond to have more double bond character to what is expected, in the products the sp3 bond lengths match the standard values expected as there is no double bond conjugation. Sp2 C-C bond lengths in the reaction match the expected bond length.
The Van der Waals radius of the C atom is 1.7 angstroms[5]. The bonds being formed in the transition state are within the van der waals radii of the two C atoms which means that these C atoms must have an bonding interaction between them which is what would be expected of the single C-C bond being partially formed in the transition state.
Bond vibrations
The formation of the two bonds is synchronous. This is illustrated by the vibration below which is the vibration caused by the imaginary frequency on the transition state, this is the expected vibration as the orbitals involved in this bond forming interact in phase in the same manner and the overall reaction is concerted so the bonds are expected to be formed simultaneously.
Bond forming vibration |
Relevant files
Below are files used for the analysis that were not included in the above Jmol images:
Exercise 2: Reaction of cyclohexadiene and 1,3-Dioxole
Reaction overview
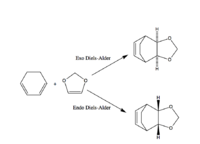
This system can undergo two different Diels-Alder reactions, the exo Diels-Alder or the endo Diels-Alder depending on the orientation of diene to the dienophile. Each Diels-Alder will have a slightly different transition state and product and as a result a different activation free energy and free energy of reaction.
This reaction had its MO's analysed to see if the same HOMO-LUMO interactions are present and if any secondary orbital had any effect on the transition state.
Thermochemistry data was also obtained and analysed to compare the two Diels-Alder reactions and to see which reaction was kinetically fastest and which product was more stable.
The analysis was carried out by optimising the products, reactants and transition states to the B3LYP/6-31G(d) level to visualise the MO's and to obtain the thermochemistry values. In addition an IRC was taken in order to obtain the electron demand of the reaction.
MO diagram and relevant transition state MO's
This Diels-Alder reaction has a slightly different MO diagram to the previous reaction analysed. This reaction is an inverse electron demand Diels-Alder due to the dienophile HOMO strongly interacting with the LUMO of the diene, this is due to the dieneophile being electron rich when compared to the diene, in the reaction analysed in exercise 1 the inverse was the case. The same orbitals are involved with each other however and so the same 4 MO's are present in the transition state, the MO's are also in the same order as even though the LUMO of the diene and the HOMO of the dienophile have a larger splitting associated with them they will still be the HOMO and LUMO of the transition state as the other orbitals also interact strongly, this matches the MO's obtained of the transition state. The diagram below only shows the primary orbitals involved in the transition state, any secondary orbital interactions are not included for clarity of the diagram but are discussed later.
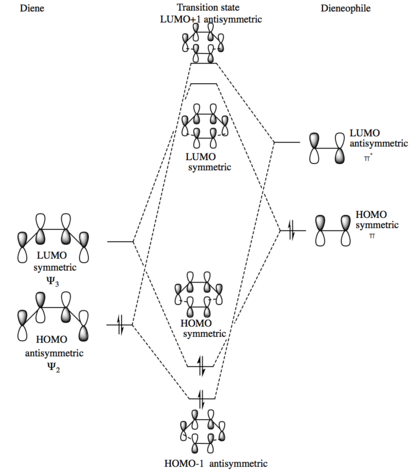
|
|
|
|
|---|
|
|
|
|
|---|
Thermochemistry
Below is a table summarising the reaction energy and the reaction barrier free energies of the exo and endo Diels-Alder reactions.
| Reaction energy (Hartrees) | Reaction energy (KJ/mol) | Reaction barrier (Hartrees) | Reaction barrier (KJ/mol) | |
|---|---|---|---|---|
| Exo Diels-Alder | -0.028156 | -73.92 | +0.06 | +157.53 |
| Endo Diels-Alder | -0.029526 | -77.52 | +0.057016 | +149.70 |
The results show that the endo Diels-Alder has a lower reaction barrier and a lower reaction energy and thus is more favoured under both kinetic and thermodynamic conditions. The reaction barrier is lower due to the secondary orbital interactions stabilising the transition state in the endo form, these interactions are not present in the exo form. The endo product has a lower reaction energy as there is less steric clash present between part of the cycloheaxdiene and the 1,3-dioxole as in the endo product these two parts are on opposite faces while in the exo form they are both on the same face of the molecule.
17:49, 17 November 2017 (UTC) You say youn have done it with b3LYP but these energies are at PM6
Secondary orbital interactions
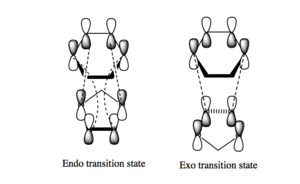
The diagram on the right shows the differences between the HOMO's of each of the two transition states. In the endo HOMO there are secondary orbital interactions present, these secondary interactions between the oxygen p orbitals and the p orbitals on the diene stabilise the overall energy of the HOMO thus lowering the reaction barrier and therefore making this form much more kinetically favourable. This orbital analysis matches the thermochemistry data obtained from the table above.
There are no secondary interactions present in the exo form as the oxygen atoms are too far away spatially to react with the diene thus not causing a stabilising effect to be present in this transition state.
These secondary interactions can also be seen in the HOMO's present above of the endo and exo transition states.
Nf710 (talk) 18:06, 17 November 2017 (UTC) This is a nice section. Nice diagrams and nice explanation of the SOO and the electron demand of the reaction. shame about the Energies being PM6
Relevant files
Below are files used for the analysis that were not included in the above Jmol images:
Exercise 3: Diels-Alder vs Cheletropic
Reaction overview
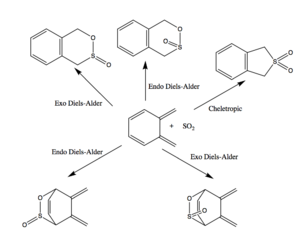
This reaction involves o-xylyene reacting with SO2 in either a Diels-Alder reaction at one of two sites or a Cheletropic reaction occurring.
IRC's were taken of one set of Diels-Alder reactions and the Cheletropic reaction in order to compare the different reaction pathways and the changes to the structure over the course of the reaction.
Thermochemistry data was also analysed for all of the reactions so the free energies of activation and reaction could be compared in order to identify the thermodynamic and kinetic products and if all of the could occur easily. This data was then visualised as a reaction profile diagram.
The analysis was carried out by optimising the products, reactants and transition states to the PM6 level to obtain the thermochemistry values. In addition IRC's were taken to visualise the reaction coordinate and to analyse how the bonding changes over the course of the reaction.
IRC's
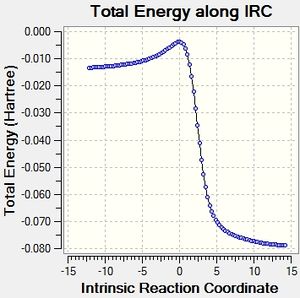
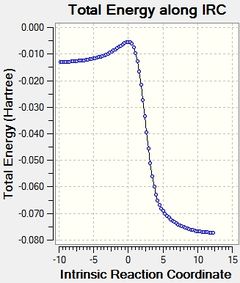
Below are animations of the IRC's of the 3 initial reactions analysed and the reaction pathways for all of them.
Click to animate the above IRC's.
The IRC's show that in all 3 of the reactions the products are significantly more stable than the reactants, all of the activation barriers in addition seem to be low enough to allow all of these reactions to be feasible reactions. The gif's show for the two Diels-Alder reactions that the same reaction pathway is followed for both, the only difference is the position of one of the oxygens on the SO2 molecule. Meanwhile the gif for the Cheletropic reaction shows the SO2 molecule approach from a different trajectory, this means that the trajectory the SO2 molecule approaches from affects the type of reaction it undergoes with xylyene.
Thermochemistry and reaction profile diagram
Below is a table summarising the reaction energy and the reaction barrier free energies of the exo and endo Diels-Alder reactions and the Cheletropic reaction.
| Reaction barrier (Hartrees) | Reaction barrier (KJ/mol) | Reaction energy (Hartrees) | Reaction energy (KJ/mol) | |
|---|---|---|---|---|
| Exo Diels-Alder | +0.032422 | +85.12 | -0.0382 | -100.29 |
| Endo Diels-Alder | +0.030904 | +81.14 | -0.03795 | -99.64 |
| Cheletropic | +0.039407 | +103.46 | -0.059657 | -156.63 |
(The 'kilo-' prefix is small 'k' Tam10 (talk) 09:55, 13 November 2017 (UTC))
These findings match the IRC graphs above, they show that the Cheletropic reaction is the preferred thermodynamic product but it has the largest reaction barrier and so it will only form as the major product in thermodynamic conditions. Meanwhile the endo Diels-Alder is preferred over the exo Diels-Alder due to its marginally smaller reaction barrier which is caused by secondary orbital interactions, the reaction energies are approximately the same due to a very minor difference in the final product formed which means in kinetic conditions the endo Diels-Alder will be formed over the other two products. This information is all visualised below on a simple energy profile diagram.

(This diagram is good, but you could improve the clarity at the TS. Avoid bunching text together if possible Tam10 (talk) 09:55, 13 November 2017 (UTC))
Structural changes over the reaction
During the course of the reaction the 6-membered ring bonding turns from a diene being present in the ring into a ring with equal bond lengths present throughout and hence the electron density is distributed evenly around the ring like benzene, the bond lengths are all equal and are of intermediate length between that of a single and double bond. Producing a benzene ring will greatly stabilise the product and therefore make it stable unlike xylylene, it also explains why these reactions can occur.
Second Diene fragment analysis
o-xylylene also has a second diene fragment that can undergo endo and exo Diels-Alder reactions, below is a table summarising the reaction energy and the reaction barrier free energies of the exo and endo Diels-Alder reactions.
| Reaction barrier (Hartrees) | Reaction barrier (KJ/mol) | Reaction energy (Hartrees) | Reaction energy (KJ/mol) | |
|---|---|---|---|---|
| Exo Diels-Alder | +0.0454 | +119.20 | +0.007651 | +20.09 |
| Endo Diels-Alder | +0.042415 | +111.36 | +0.005906 | +15.51 |
As the free energy of both products is larger than that of the reactants so this Diels-Alder reaction is very unlikely to occur. In addition, the reaction barriers are larger than any of the previous reactions analysed which further makes this reaction unlikely. The reaction is so unlikely because the benzene ring which forms in the previous reactions to produce the products does not form and therefore cannot stabilise the products. The endo form will be preffered slightly due to le
(Pretty risky writing incomplete sentences to come back to without placeholders! Fortunately a complete discussion isn't needed for this section Tam10 (talk) 09:55, 13 November 2017 (UTC))
Relevant files
Below are the files used for analysis:
Reactants File:MeaREACTDIENE3.LOG File:MeaREACTDIENEOPHILE3.LOG
Transition states File:Meaex3CHELTS2PM6.LOG File:MeaEX3TSENDOOPTPM6.LOG File:Meaex3tsexopm6.LOG
Products File:Meaex3cheleoprod.LOG File:Meaex3EEXOPROD.LOGFile:Meaex3endoPROD2.LOG
Transition states diene 2 File:Meaex3diene2tsendo.LOG File:Meaex3diene2exots.LOG
Products diene 2 File:Mea3ex3diene2endoprod.LOG File:Meaex3diene2exoprod.LOG
Conclusion
The above exercises shows that Diels-Alder reactions can be analysed very well with computational methods; transition states, reaction pathways, molecular orbitals and relative reactivities can all be determined and analysed easily from the initial potential energy surface using both semi empirical and DFT methods. The information obtained can also be related back to the expected theory very well as the theory matches what is achieved computationally. This means that for Diels-Alder reactions computational techniques can be employed to predict reactivity and final structures, to give a couple of examples, before the experiment is carried out in a lab, this would prevent reactions which will not produce the product anticipated from being carried out in a lab, thus saving time and money on chemicals.
Overall computational methods have been shown to be effective for this class of reaction and should be employed for further analysis of Diels-Alder reactions. However all the reactions explored were quite simple so computational methods may not be quite as effective for larger molecules, this would need to be investigated. Other types of pericyclic reactions could also be investigated computationally to see if this type of analysis is just as effective for them.
References
- ↑ https://chemistlibrary.files.wordpress.com/2015/02/modern-quantum-chemistry.pdf pages 111-115
- ↑ http://www.cup.uni-muenchen.de/ch/compchem/energy/semi1.html
- ↑ https://link.springer.com/article/10.1007%2Fs00894-007-0233-4#Sec2
- ↑ https://ocw.mit.edu/courses/chemistry/5-12-organic-chemistry-i-spring-2003/lecture-handouts/04.pdf
- ↑ http://periodictable.com/Properties/A/VanDerWaalsRadius.v.html