Rep:Mde14tscomp
Year Three computational lab - Transition Structures
Michael Edwards - 00940539
Introduction
Computational methods can be used to measure potential energy surfaces, which are defined as mathematical functions that calculate the energy of molecules as functions of their geometries and degrees of freedom of movement. Through surface calculations it is possible to find energy minima and maxima, which can correspond to the optimised product of a reaction, the optimised geometry of a molecule, or a transition state within a reaction. Energy minima can be determined as stationary points, which have zero gradient and a positive second derivative of the function, which is a minimum point in standard calculus. Transition states are stationary points with a zero gradient and a negative secondary derivative of the function used, which is a maximum point in calculus. Computations at these transition state structures are shown to yield negative, or imaginary vibrations in a frequency calculation that correspond to the reaction path between the reacting atoms or functional groups. This can be observed by visualising and animating the negative vibration.
The Gaussian computer program can be used to calculate potential energy surfaces to varying degrees of precision dependent on the methods used. In this experiment, a mixture of the semi-empirical PM6 method and the Density Functional Theory B3LYP/6-31GD method were employed to carry out calculations along potential energy surfaces for a number of cycloaddition reactions and alternative proposed reactions. The cycloadditions under investigation are between ethene and butadiene, cyclohexadiene and 1,3-dioxole, and xylylene and sulfur dioxide. Transition states, which occupy stationary points on the potential energy surface, have been determined for all of the cycloadditionss, alongside an investigation of the reaction pathways along the Intrinsic Reaction Co-ordinate. Thermochemical data was attained from these calculations but is of limited use due to lack of correction of multiple degrees of freedom.
Nf710 (talk) 21:44, 12 January 2017 (UTC) This section is vague. You should be talking about co-ordinates and higher dimensions when decribing stationary point. The degrees of fredom of the molecule are the number od dimensions of the PES. a TS onyl have one of thiese with negative curvature.
Exercise One - Cycloaddition of Ethene and Butadiene

Method
The semi-empirical PM6 method was used to optimise the reactant molecules. A 'guess' transition state was employed, with the reacting atomic termini for the reactants frozen for an initial energy optimisation then unfrozen to determine the transition state. An Intrinsic Reaction Co-ordinate calculation was carried out in both forward and reverse directions to illustrate the full reaction.
Molecular orbitals of the reactants and the transition state
For a standard Diels-Alder reaction, the following molecular orbital diagram illustrates the reacting orbitals in the cycloaddition transition state. The HOMO and LUMO+1 of butadiene, as well as the LUMO of ethene, are antisymmetric, and the HOMO-1 and LUMO of butadiene and the HOMO of ethene are symmetric.

MOs have been visualised in GaussView for the orbitals under consideration in this MO diagram. The HOMO and LUMO for the reactant molecules, and the four molecular orbitals these produce in the transition state, can be seen here.
It can be clearly seen that the orbitals predicted to react by symmetry are the ones that react, namely, the HOMO of ethene and the LUMO of butadiene, and the LUMO of ethene and the HOMO of butadiene. It is therefore possible to conclude that the reaction is ‘allowed’ when the symmetry of the reacting orbitals is the same, as per standard molecular orbital theory, and ‘forbidden’ when the orbitals that need to react do not share the same symmetry label (in this case, symmetric or antisymmetric).
Given that an interaction between orbitals is seen to occur when the symmetry labels are the same, it can be stated that the orbital overlap integral is non-zero for symmetric-symmetric and asymmetric-asymmetric interactions, and zero for symmetric-asymmetric interactions.
21:51, 12 January 2017 (UTC) INteractions betweeen the ornitalls allowed. not the reaction.
Change in Carbon Bond Lengths within the Reactants and Transition State to form the Product
Carbon bond lengths were investigated for the PM6-optimised reactants (namely, ethene and butadiene), the transition state of the Diels-Alder reaction between the two (as determined by PM6 calculation on Gaussian), and the PM6-optimised product, cyclohexene. The changes in bond lengths can be observed in the graph figure. Bond lengths are clearly shown to change throughout the reaction as the cycloaddition reaction between ethene and butadiene occurs. The single bond in the butadiene fragment is seen to shorten as it gains more sp2 character and becomes a double bond, and the opposite is observed with the double bonds. In the ethene fragment, the bond is seen to lengthen as the bond gains more sp3 character and becomes a single bond. The internuclear distance between the fragments is observed to change significantly from the transition state at 2.115 Å, to the product at 1.54 Å.
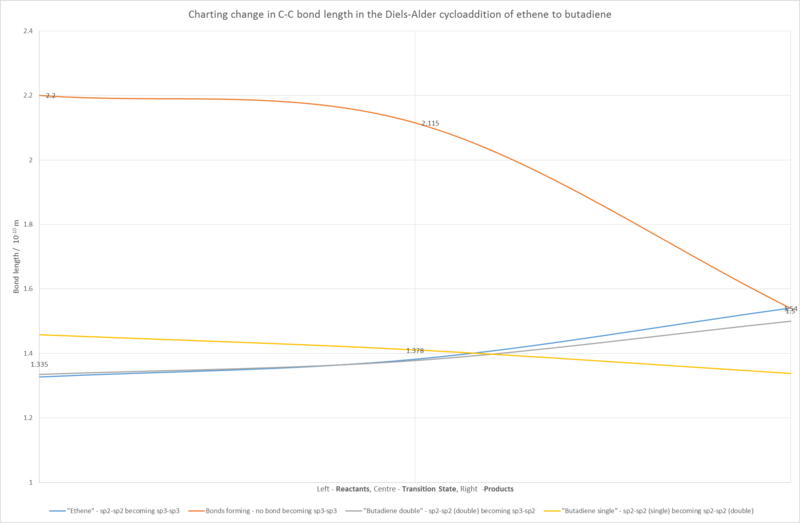
| C-C bond | Reactants (frozen termini) | Transition State | Product |
|---|---|---|---|
| C-C in ethene molecule | 1.327 | 1.382 | 1.541 |
| C-C between ethene and butadiene (form during reaction) | 2.200 | 2.115 | 1.54 |
| C-C in butadiene double bonds | 1.335 | 1.378 | 1.500 |
| C-C in butadiene single bond | 1.458 | 1.411 | 1.338 |
When considering typical single and double C-C bonds, which are 1.54 Å and 1.34 Å respectively [2], it is clear that the transition state has bonds that exhibit a blend of single and double bond characters, due to their intermediate lengths between the two lengths typically observed. The Van der Waals radius of C is 1.70 Å [3], which is longer than the single and double bonds observed in the product (as expected with the overlap of VdW surfaces required for the formation of bonds) showing that the partially formed C-C bonds in the TS are in the early stage of formation. However, since the atoms are much closer than the sum of two Van der Waals radii of carbon (2.11 Å as opposed to the expected 3.4 Å), it is clear that the minimum point of the transition state is in a state of bond formation between the two reactants.
IRC and Transition State Vibrational Frequency
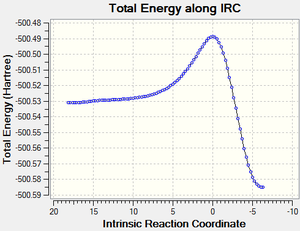
The formation of the two bonds is seen to be a concerted process from the IRC calculation of the reaction. It is observed from the vibration that corresponds to the reaction path in the transition state (the imaginary vibration at - 948 cm-1) that this process occurs such that the bonds are formed at the same time in a synchronous process. The lowest positive frequency in the product can be seen to support this judgment, since the frequency corresponds to a totally symmetric vibration of the formed bonds.
Calculation Log files - Exercise One
| PM6 optimised transition state between butadiene and ethene
| PM6 optimised product - cyclohexene
Exercise Two - Cycloaddition of cyclohexadiene and 1,3-dioxole - Formation and analysis of Endo and Exo Products

22:28, 12 January 2017 (UTC) These dagrams ar wrong if yiu rotate each one 180 degrees you obtain the saame again.
Method
The semi-empirical PM6 method was used to optimise the reactant molecules, which were reoptimised using the B3LYP-631GD method. A 'guess' transition state was employed, with the reacting atomic termini for the reactants frozen for an initial energy optimisation then unfrozen to determine the transition state at first PM6 and then B3LYP-631GD level. Since two products were possible from the interaction between the orbitals in the cycloaddition, both were considered in this section.
Molecular Orbitals in the Transition States for the exo and endo products
Employing the MO diagram for the Diels-Alder cycloaddition of ethene and butadiene used in Exercise One, it is possible to infer the MOs for this interaction by symmetry, since the fragments in the interaction are similar to those used previously.
The transition state for both the endo and exo interactions has been located - a stationary point has been identified with a negative or imaginary frequency corresponding to the reaction path along the Intrinsic Reaction Co-ordinate. Both of these vibrations have been animated below.
| Endo | Exo |
|---|---|
 |
 |
MOs for the Diels-Alder cycloaddition between 1,3-dioxole and cyclohexadiene have been visualised using GaussView. The symmetric nature of these orbitals means that the ‘side-on’ view of the orbitals as provided in the images is sufficient to observe the orbital interactions within the transition states.
(In your diagrams, try to make the symmetry clearer. It's just about visible by looking at the edges of the surfaces. Aim to write a report so that a chemist who isn't familiar with the topic can understand it. Tam10 (talk) 11:49, 3 January 2017 (UTC))
Diels-Alder reactions can be classified as either ‘normal’ or ‘inverse’ demand with regards to the electronic interaction occurring. Given the nature of the dienophile within this reaction, namely 1,3-dioxole, it can be inferred from theory that the electron-donating behaviour of the two oxygen atoms into the π system means that this is an inverse-demand Diels-Alder reaction [4], which is confirmed by a quick analysis of the HOMO of both endo and exo transition states, which demonstrate the reaction of the dienophile HOMO with the diene LUMO to form the HOMO of the transition state.
Nf710 (talk) 22:34, 12 January 2017 (UTC) correct conclusion
Thermochemistry and Reaction Energies - Differences between the exo- and endo- products formed
Disclaimer - the data used here is likely subject to large errors due to calculation error.
The calculations for the transition states and products can ideally be used to determine the kinetically and thermodynamically favoured product of the reaction under investigation, by considering the reaction barrier energies and the energy minimum of the formed products from their respective transition states.
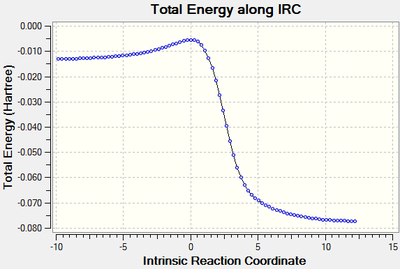
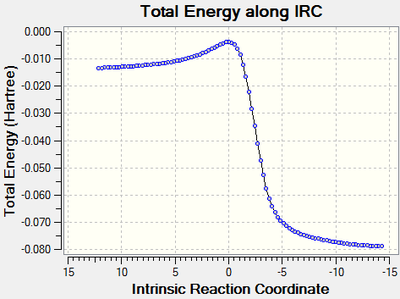
An Intrinsic Reaction Co-ordinate calculation was carried out for the formation of the exo and endo products to provide energy profiles for each reaction - these are contained below. The Gibbs free energies are considered in the log files for each of the calculations. The activation energy, reaction barrier, or EA, is taken to be the energy difference between the first IRC point on the left and the transition state IRC, and the change in energy of reaction, or ΔGr, is taken to be the energy difference between the first and last IRC points.
(This must have taken a very long time to calculate! For the sake of time, we ask for IRCs to be calculated with PM6 and the stationary points reoptimised with B3LYP Tam10 (talk) 11:49, 3 January 2017 (UTC))
(Reaction barriers and activation energies here are given for the electronic energy only. You are neglecting thermal and zero energy point corrections to the energy, as well as symmetry issues at reactants/products Tam10 (talk) 11:49, 3 January 2017 (UTC))
The two calculations start at approximately the same energy, and a clear difference can be observed between the first IRC and the transition state IRC of the reactions - this is an energy difference of 10.5 kJ mol-1, arising due to circumstances to be covered later. When considering the overall change in energy, the observed energy difference is once again 10.5 kJ mol-1, and suggests that the endo product is the thermodynamically favoured product in this reaction, whereas the exo product is the kinetically favoured product. Repeats of this calculation yield the same result, but investigation of the wider literature suggests that typical Diels-Alder reactions yield the exo product as the thermodynamic product and the endo product as the kinetic product [5], which is against what is observed here and suggests some part of the calculation have not worked correctly. However, since these reactions go to the expected products, in particular through a minimum that can be easily identified to be a suitable transition state, at least some part of the calculations must have been correct.
(You've shown that the barrier is lower for endo - doesn't it make endo the kinetically favoured product? Tam10 (talk) 11:49, 3 January 2017 (UTC))
Secondary Orbital Effects and Sterics - differences in exo and endo products
Secondary orbital interactions can be observed between p orbitals on the oxygen atoms in the 1,3-dioxole and the π system of orbitals of the diene in the endo product that reduce the energy of the transition state, making it the more favourable reaction pathway with a lower reaction barrier energy. Comparatively, no such secondary orbital interaction exists in the exo transition state structure as the orbital interaction is blocked by the presence of the cyclic alkyl chain, which is a steric factor hindering the interaction. This results, as seen above, in a clear energy difference between the endo and exo transition state. These orbitals can be seen in the table in the MO sub-section of this exercise.
Calculation Log files - Exercise Two
Note - unable to remove the negative frequency on the 1,3-cyclohexadiene due to symmetry considerations.
(Imaginary frequencies can be removed by breaking symmetry and repotimising Tam10 (talk) 11:49, 3 January 2017 (UTC))
| B3LYP-6-31G-D optimised 1,3-cyclohexadiene
| B3LYP-6-31G-D optimised 1,3-dioxole
| B3LYP-6-31G-D optimised transition state - ENDO
| B3LYP-6-31G-D optimised product - ENDO
| B3LYP-6-31G-D optimised transition state - EXO
| B3LYP-6-31G-D optimised product - EXO
Nf710 (talk) 22:45, 12 January 2017 (UTC) You should have shown the SSO with a diagram and you got your kenetic and thermo products mixed up.
Exercise Three - Xylylene and SO2 - Diels-Alder vs Cheletropic
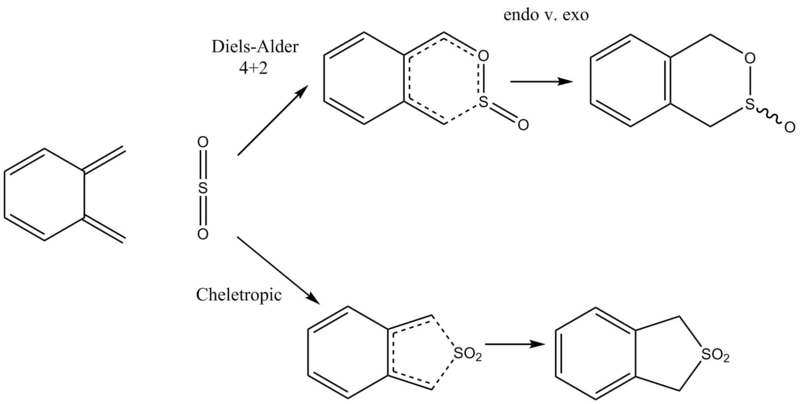
Method
The semi-empirical PM6 method was used to optimise the product molecule, after which the symmetry of the lowest vibrational frequency was broken and the structure reoptimised. Bonds between the xylylene and SO2 fragments were broken and the transition state was determined using a PM6 calculation. An IRC calculation for each of the endo, exo and cheletropic reaction was run at the PM6 level.
IRC and Energy Profiles for the Diels-Alder reactions (endo and exo) and cheletropic reaction between xylylene and SO2
The transition states were identified - negative frequencies exist in each of the optimised structures corresponding to the reaction pathway along the IRC.
| Endo | Exo | Cheletropic |
|---|---|---|
 |
 |
 |
Values for activation and reaction energies were determined from these graphs in the same way as in Exercise 2.
To better illustrate this data, the figure below displays the collated graphs from above. It clearly shows the cheletropic transition state as being of the highest energy, but also with the lowest energy minimum for its corresponding product, suggesting that it is a thermodynamically more favoured product than the Diels-Alder reactions, which in this case form the 'kinetic' products.
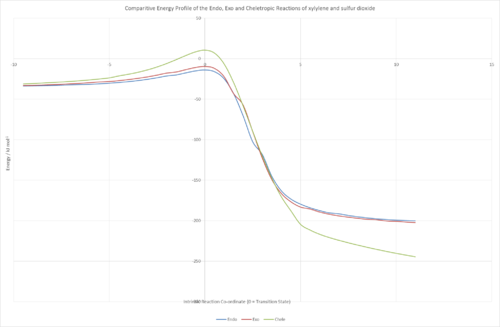
(Be careful when plotting data like this. Their x values are different, which is why you are getting bumps throughout Tam10 (talk) 11:49, 3 January 2017 (UTC))
Within the data for Diels-Alder reactions, it can be observed that the endo transition state is lower in energy, and its product is of higher energy, than the exo equivalent, meaning that the endo product is kinetically favoured and the exo product is thermodynamically favoured. All the reactions are found to be exothermic with regards to change in enthalpy and free energy, and, due to the latter, will all readily occur at STP, the standard condition used for the calculations.
Due to the same conditions as in Exercise 2, the significant energy difference between the exo and the endo transition states means that the endo product is favoured overall because it forms fastest and most readily. The difference in energy is down to a secondary orbital interaction between the π system on the deforming xylylene and the π system on the S=O that either aligns downwards, overlapping with the other π system, or upwards away from the other orbitals.
The instability of the xylylene fragment and alternative rearrangement
One of the reasons behind the favourability of this interaction and subsequent reaction is the establishment of aromaticity in the benzene ring within the forming molecule - this is a major driving force for all of the interactions under investigation in this exercise. Xylylene is found to be an unstable molecule that also readily undergoes this alternative reaction to establish the aromaticity in the benzene ring - this was found by an alternative calculation to determine whether the angle of approach affected the cheletropic reaction, which also discovered that without the specific geometry required and shown in the IRC, the competing cyclisation occurs in competition. The log file for this can be found in the log file section.
(You've left the S-C bonds frozen in this calculation, essentially holding them apart. This can lead to strange things, such as what you've got. Freezing bonds should be used to generate a guess structure (close to a stationary point), which is then reoptimised Tam10 (talk) 11:49, 3 January 2017 (UTC))

Calculation Log files - Exercise Three
| PM6 optimised transition state for the Diels-Alder exo product
| PM6 optimised transition state for the Diels-Alder endo product
| PM6 optimised transition state for the cheletropic reaction product
| PM6 Intrinsic Reaction Co-ordinate calculation for the endo transition state and product
| PM6 Intrinsic Reaction Co-ordinate calculation for the exo transition state and product
| PM6 Intrinsic Reaction Co-ordinate calculation for the cheletropic transition state and product
References
- ↑ Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Butadiene_Ethene_Cycloaddition123.png, accessed 14/12/2016
- ↑ Texas A&M University Chemistry Department, http://www.chem.tamu.edu/rgroup/connell/linkfiles/bonds.pdf, accessed 14/12/2016
- ↑ PeriodicTable, http://periodictable.com/Properties/A/VanDerWaalsRadius.v.log.html, accessed 14/12/2016
- ↑ Organic Chemistry Portal, http://www.organic-chemistry.org/namedreactions/diels-alder-reaction.shtm, accessed 14/12/2016
- ↑ University of Liverpool, http://www.chemtube3d.com/DAendo_vs_exo,cyclopentadiene_and_maleic_anhydride.html, accessed 15/12/2016