Rep:MOD:spk15TSEx3
Exercise 3: Diels-Alder vs Cheletropic
Reaction Scheme
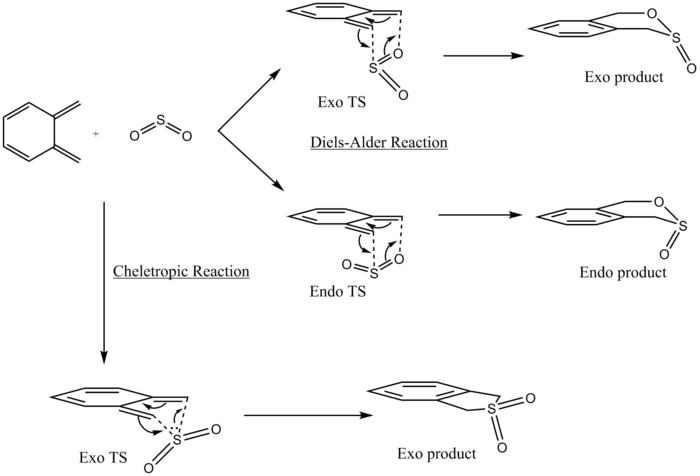
O-Xylylene can react with SO2 via 2 different pathways- Diels-Alder reaction or Cheletropic reaction shown in Figure 11 . The Diels-Alder reaction produces a mixture of endo and exo products while the Cheletropic reaction only produces a single exo product due to the endo pathway being very kinetically unfavoured.
(I think there is only one cheletropic (two if not counting symmetry Tam10 (talk) 14:41, 31 January 2018 (UTC))
Jmol Files
| Reactants | Transition States | Product | ||||||
|---|---|---|---|---|---|---|---|---|
O-Xylylene |
Diels-Alder Exo TS |
Diels-Alder Exo product | ||||||
SO2 |
Diels-Alder Endo TS |
Diels-Alder Endo product | ||||||
Cheletropic TS |
Cheletropic product |
IRC Pathway
| Endo TS | Exo TS | Cheletropic |
|---|---|---|
 |
 |
|
According to Huckel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with pair electrons. This is true of aromatic compounds, which display extremely high stability. With aromatic compounds, 2 electrons fill the lowest energy MO, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted n), leaving all bonding orbitals filled and no anti-bonding orbitals occipied. This gives the Huckel Rule that compounds with a total of [4n+2] π electrons have higher stability and compounds with [4n] π electrons display anti-aromatic behaviour and have a lower stability since their anti-bonding orbitals are now filled.10 The initial O-xylylene molecule has 8π electrons delocalised across the 4 conjugated double bonds. Hence, the molecule is anti-aromatic and highly unstable. Throughout the course of the reaction, the 6-membered ring in O-xylylene forms a benzene ring. Benzene has 6 π electrons and is aromatic and stabilised. Hence, this reaction proceeds favourably to form an aromatic product. This also explains why o-xylylene is so reactive as it is anti-aromatic and highly unstable.
Reaction Dynamics
(Maybe best to call this section "Reaction Thermodynamics" Tam10 (talk) 14:41, 31 January 2018 (UTC))
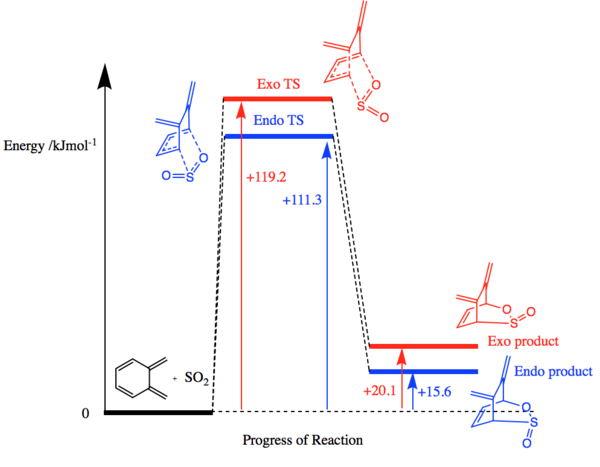
Similar to exercise 2, the Gibbs Free energies were calculated at both the PM6 and B3LYP level for the reactants, products and transition states and are shown in Figure 12. The activation energy (reaction barrier) and reaction energies were also calculated using the same formula as in exercise 2. They are shown in Figure 13.
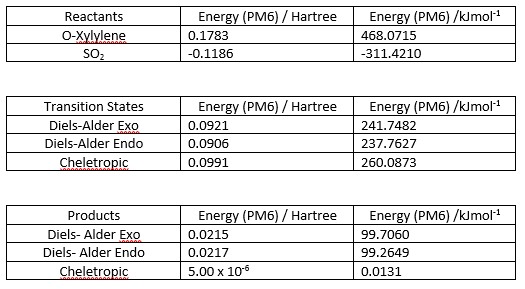
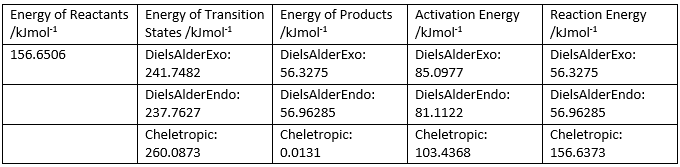
(If you did B3LYP calculations, why not show the results of them too? Seems like wasted effort otherwise Tam10 (talk) 14:41, 31 January 2018 (UTC))
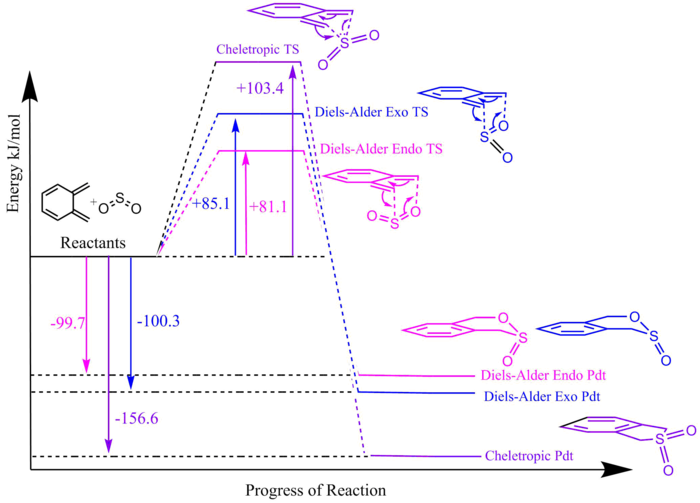
(Nice diagram, but it might be confusing having the x-axis somewhere other than y=0 Tam10 (talk) 14:41, 31 January 2018 (UTC))
Alternative Diels-Alder Reaction
SO2 can also react with the second cis-butadiene fragment in O-Xylylene according to the reaction scheme shown in Figure 15.

| Transition States | Product | ||||
|---|---|---|---|---|---|
Endo TS |
Endo product | ||||
Exo TS |
Exo product |
Due to the this second cir-butadiene fragment being much more sterically hindered, there is a much larger activation energy to reach this transition state. Furthermore, the product formed is higher in energy than the reactants, making the endothermic reaction very thermodynamically unfavourable.