Rep:MOD:spk15TSEx2
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Scheme
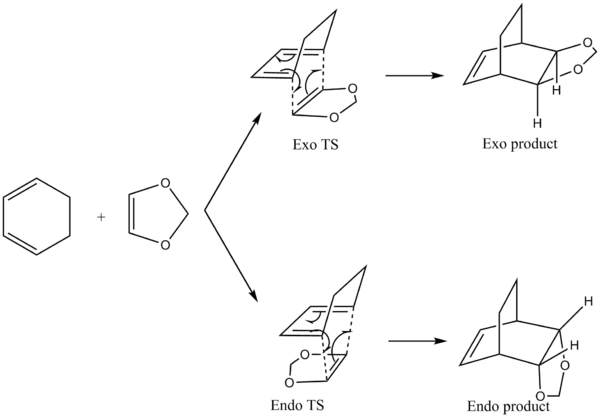
Cyclohexadiene reacts with Dioxole in a concerted, suprafacial [4+2] cycloaddition according to the mechanism shown below in Figure 5 to form two products- the exo and endo products.
Jmol Files
| Reactants | Transition States | Product | ||||||
|---|---|---|---|---|---|---|---|---|
Cyclohexadiene |
Exo TS |
Exo product | ||||||
Dioxole |
Endo TS |
Endo product |
Single Point Energy Calculation
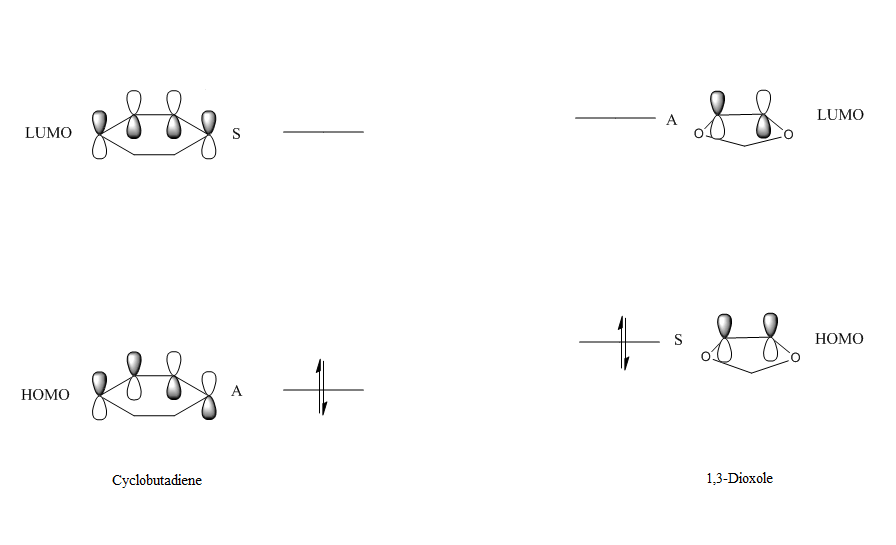
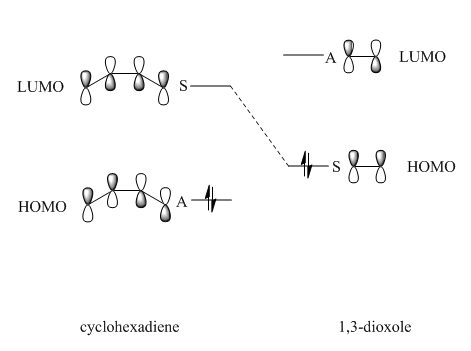
In order to determine if the reaction between cyclohexadiene and 1,3-dioxole proceeded via normal or inverse electron demand, the relative levels of the HOMO and LUMO of the reactants were compared by performing a single point energy calculation. This allowed the reactants to be studied on the same potential energy surface and the energies of their MOs to be compared more accurately. In this reaction, the HOMO of 1,3-dioxole, the dienophile, was found to be higher than the HOMO of cyclohexadiene, the diene, as shown below. This indicates the reaction proceeds via inverse electron demand.
(Fv611 (talk) Good discussion here)
| Cyclohexadiene | Relative Energies of the HOMO and LUMO of the Reactants | 1,3-Dioxole | ||||
|---|---|---|---|---|---|---|
 |
||||||
MO Analysis of Reaction
(Fv611 (talk) This MO diagram is very good, but you are still missing the symmetry labels for the TS MOs. Also, you have not discussed at all the difference between the endo and exo case in terms of MO relative energies.)
| Occupied Orbital | HOMO | LUMO | Unoccupied Orbital | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exo | ||||||||||||
| Endo |
| Cyclohexadiene | MO diagram for the formation of the Cyclohexadienediene/1,3-Dioxole transition state | 1,3-Dioxole | ||||
|---|---|---|---|---|---|---|
 |
||||||
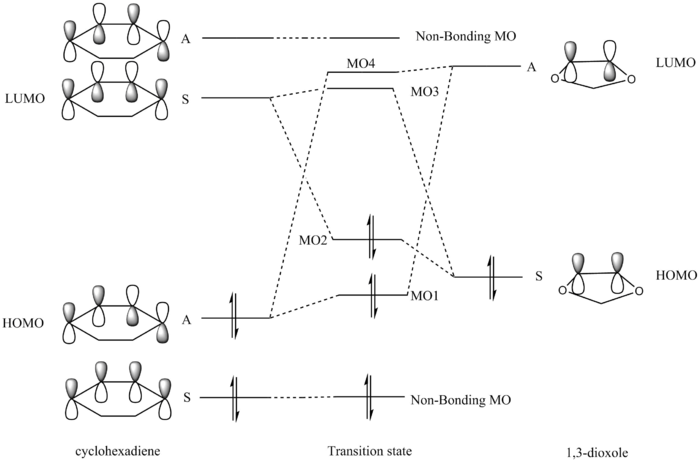
In this inverse electron demand [4+2] cycloaddition reaction, the 1,3-dioxole acts as a electron rich dienophile since the oxygen atoms can donate their lone pair of electrons into the pi system of the double bond. Hence, the HOMO of the 1,3-dioxole is raised higher than the HOMO of the dienophile and is high enough in energy to interact with the LUMO of the diene (cylohexadiene). MO1 and MO4 are a bonding/antibonding pair formed from the overlap of the HOMO of cyclohexadiene and the LUMO of the 1,3-dioxole. MO2 and MO3 are another bonding/antibonding pair formed from the overlap of the HOMO of the 1,3-dioxole and the LUMO of cyclohexadiene.6
| Normal | Inverse |
|---|---|
 |
 |
Reaction Dynamics
The Gibbs Free energies of the reactants, products and transition states were tabulated at the PM6 and B3LYP level, and are shown in Figure 6 below.
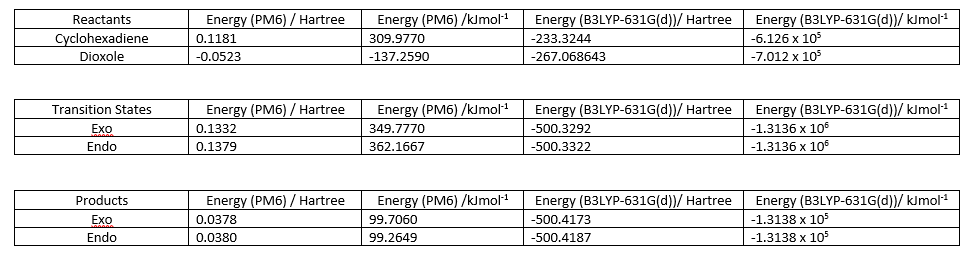
The reaction energy and reaction barrier were calculated according to the equations shown below:
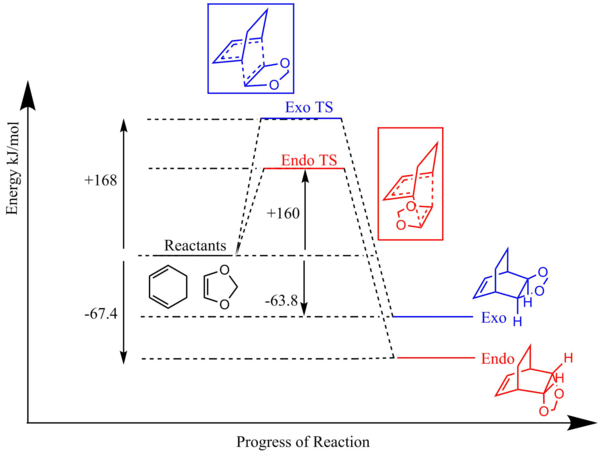
According to the reaction profile (Figure 7), the endo product is both kinetically and thermodynamically favoured. The endo activation energy is lower than the exo activation energy which means that the reactants will form the endo transition state faster than the exo transition state. The endo transition state is lower in energy than the exo transition state since there are secondary orbital interactions between the butadiene pi orbitals and the p orbitals of the oxygen atoms which is discussed more below. This lowers the energy of the endo transition state. The endo Diels-Alder product is also lower in energy than the exo product which indicates that the endo product is more stable and hence thermodynamically favoured. The endo product is lower in energy as there is greater steric clash in the exo product between the CH2 hydrogens and the oxygen atoms as shown in Figure 8 below.7 On the other hand, in the endo product with the dioxole in an axial position, there is much less steric clash between the CH2 hydrogens and the other hydrogen atoms since hydrogen has a much smaller VDW radius than oxygen. Furthermore, the endo product is more thermodynamically preferred as the former 1,3-Dioxole component is anti to the carbon bridge group rather than gauche in the exo product. There is reduced steric hindrance in the molecule as the two larger groups are anti to one another. This is shown in Figure 9.
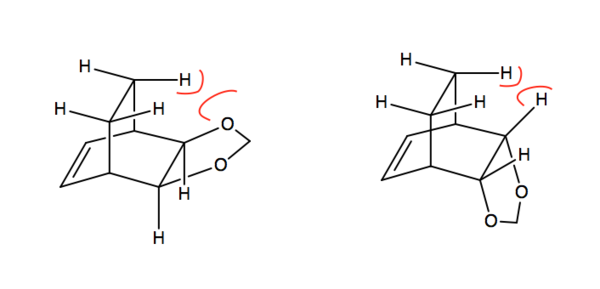
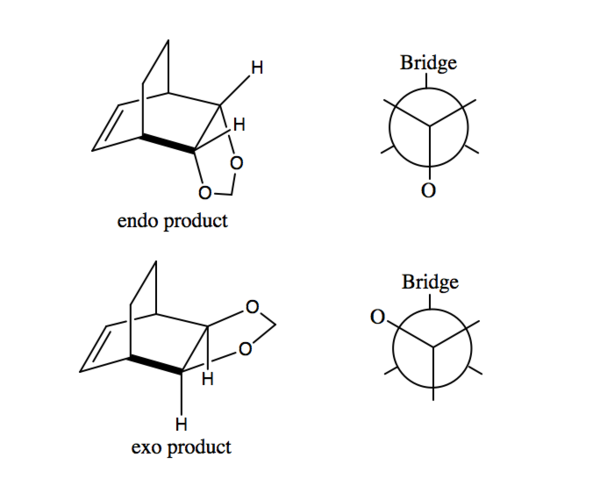
Secondary Orbital Interactions
| HOMO of Exo TS | HOMO of Endo TS | ||||
|---|---|---|---|---|---|
In both the endo and exo transition states, there are primary orbital interactions between the pi orbitals of cyclohexadiene and dioxole which result in the formation of the new sigma bonds. In the endo transition state, there are additional secondary orbital interactions between the p-orbitals of the cyclohexadiene and the non-bonding p-orbitals of the oxygen atoms in dioxole, this stabilises the transition state and results in the endo transition state forming faster, and making the endo product more kinetically favourable.8 This is shown more clearly in Figure 10 below.
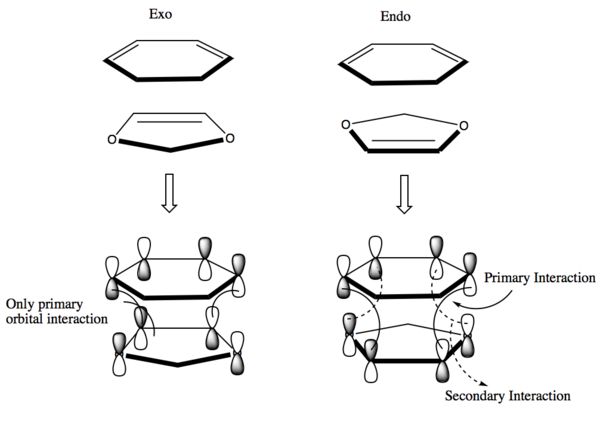
Nf710 (talk) 22:05, 8 February 2018 (UTC) This was a really good section, well done you have gone beyond the script. the part about the reactant MOs was very good.
