Rep:MOD:Yz19114
Y3C-Computational lab Transition states and reactivity
Introduction
In this computational lab session, the aim is to locate and characterise transition states of several pericyclic reaction by applying suitable methods in GaussView. Firstly several basic concepts need to be explained. A potential energy surface is a mathematical function that gives the energy of a molecule as a function of its geometry, a minimum in PES is a point at which the energy rises in all directions, and a transition state is a point at which the energy decreases along the reaction pathway. The gradients (first derivatives) of these two points are all zero but the curvature(second derivative) of a minimum is positive and the curvature of a transition state is negative because the energy is maximum at TS along the reaction pathway. And the frequency is equal to the second derivative, so a negative frequency corresponds to a transition state.
Nf710 (talk) 17:25, 4 November 2016 (UTC) Imaginary frequency, Gaussian just prints it as a negative.
Exercise 1: Reaction of Butadiene with Ethylene
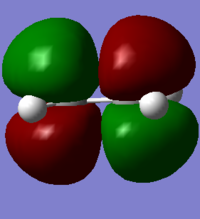
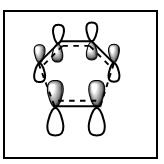
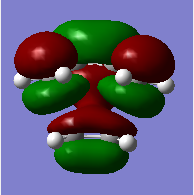
MO diagram for the formation of the butadiene/ethene TS(normal demand DA)

| HOMO | LUMO | |
|---|---|---|
| Butadiene | 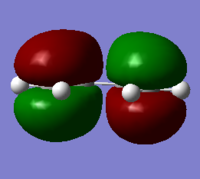
|
|
| Ethene | 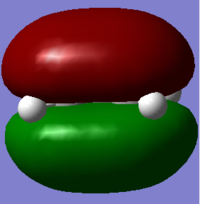
|
|
| Transiotion
State |
|
|
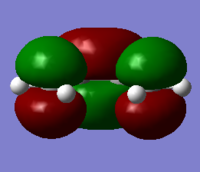
From the HOMO and LUMO diagrams above, it can be seen that the HOMO of ethene interacts with the LUMO of butadiene to give the HOMO(in-phase) and LUMO(out-of-phase) of transtion state. And it is obviously that all these orbitals are gerade, so the symmetry of two interacted orbitals should be the same when the reaction is allowed, otherwise the reaction is forbidden. The orbital overlap integral is zero for the case of a gerade-ungerade interaction and non-zero for the case of a gerade-gerade interaction and an ungerade-ungerade interaction.
| Butadiene | Ethene | Transition State | Product(cyclohexene) | |
| Bond
length ( Å) |
C12=C34=1.335
C23=1.468 |
1.327 | C12=C34=1.380
C23=1.411 C56=1.382 C16=C45=2.115 |
C12=C34=1.501
C23=1.338 C56=1.541 C16=C45=1.540 |
As reaction proresses, C12 and C34 bond lengths increases because the double bonds in butadiene breaks gradually to form single bonds, the same reason for C56 double bond in ethene. And C23 bond length decreases as a new double bonds forms gradually. Typical sp3 and sp2 C-C bond lengths are 1.54Å and 1.34Å respectively, and the Van der Waals radius of the C atom is 1.7Å.[1][2] Van der Waals radius is used to define half of the distance between the closest approach of two non-bonded atoms of a given element.[3] Partly formed C-C single bond length (C16 or C45) is 2.115 Å, which is shorter than double Van der Waals radius(3.4Å).
| TS Vibration | Lowest Positive Frequency Vibration | IRC Animation | IRC plots |
|---|---|---|---|
|
|

|
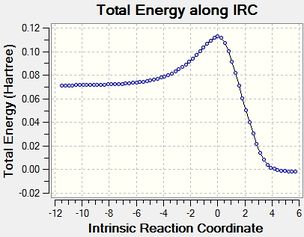
|
According to the transition state vibration diagram, it seems like the two reactants vibrate towards each other to form new C-C bonds. The IRC can prove that two new C-C single bonds formed simultaneously, and there is only one peak in the IRC plots which shows two bond are formed synchronously as well. While in the lowest positive frequency vibration diagram, the two reactants vibrate parallel to each other, which shows they are not going to react with each other and it is not the same as a TS.
Nf710 (talk) 17:37, 4 November 2016 (UTC) Good use of IRC to show that bond formation is synchronous. More detail about why this coordinate can be used to show bond formation would have been good.
Exercise 2: Reaction of Benzoquinone with Cyclopentadiene
| HOMO | LUMO | |
|---|---|---|
| Cyclopentadiene | 
|

|
| Benzoquinone | 
|

|
| Endo TS | 
|
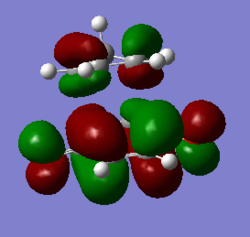
|
| Exo TS | 
|
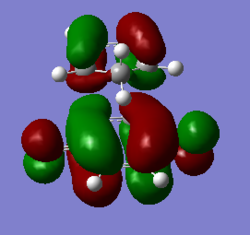
|
According to the MO diagrams, it can be seen that HOMO of dienophile(benzoquinone) interacts with LUMO of diene (cyclopentadiene) to give MOs of transition state, so it is a normal demand DA reaction. And the reason is that double bond in benzoquinone is relatively electron-rich due to the C=O electron-donating group, so the HOMO of benzoquinone has relatively high energy, which prefers to interact with LUMO of cyclopentadiene.
| Sum of electronic and thermal Free Energies(KJ/mol) | |
|---|---|
| Cyclopentadiene | -194.0348 |
| Benzoquinone | -381.3968 |
| Total Energy of reactants | -575.4316 |
| Endo TS | -575.3839 |
| Endo Product | -575.4173 |
| Endo Activation Enery | 0.0477 |
| Endo Reaction Energy | 0.0143 |
| Exo TS | -575.3813 |
| Exo Product | -575.4261 |
| Exo Activation Energy | 0.0503 |
| Exo Reaction Enegy | 0.0055 |
According to the table above, the exo product has a lower energy(-575.4261) compared with endo product(-575.4173), and the reaction energy for exo product is smaller than that for endo product as well. So the exo product is thermodynamically favourable. And the activation energy for endo product (0.0477) is smaller that for exo product(0.0503), so the endo product is kinetically favourable. Furthermore, the HOMO of endo product indicates there is secondary orbital interactions which lower the energy of TS and the activation energy as well, but there is more steric hindrance in endo TS which increases energy. Overall, the secondary orbital interactions contribute more to lower the activation energy to make endo product more kinetically favourable.
Nf710 (talk) 17:49, 4 November 2016 (UTC) Your TS energies are correct and your have presented a well balanced argument. However the energy of your endo product is wrong and therefore you havent got the correct conclusion that it is both. Very well written section.
Exercise 3: Diels-Alder vs Cheletropic
| IRC Animation | IRC Plots | |
|---|---|---|
| Diels-Alder endo product | 
|

|
| Diels-Alder exo product | 
|
|
| Cheletropic product | 
|

|
| o-xylylene | Sulfur
Dioxide |
sum of two
reactants |
DA
endo TS |
DA
exo TS |
Chel
TS |
DA endo
product |
DA exo
product |
Chel
product | |
|---|---|---|---|---|---|---|---|---|---|
| Thermal Correction(Hartrees) | 0.1312 | 0.0091 | 0.1403 | 0.1422 | 0.1421 | 0.1420 | 0.1450 | 0.1450 | 0.1452 |
| Zero Point Correction(Hartrees) | 0.1241 | 0.0057 | 0.1298 | 0.1320 | 0.1319 | 0.1316 | 0.1355 | 0.1356 | 0.1362 |
| Sum of electronic and thermal free energies(Hartrees) | 0.177945 | -0.118614 | 0.059331 | 0.090560 | 0.092077 | 0.099062 | 0.021706 | 0.021455 | 0.000002 |
| DA endo | DA exo | Chel | |
|---|---|---|---|
| Activation Energies(Hartrees) | 0.031229 | 0.032746 | 0.039731 |
| Reaction Energies(Hartrees) | -0.037625 | -0.037876 | -0.059329 |
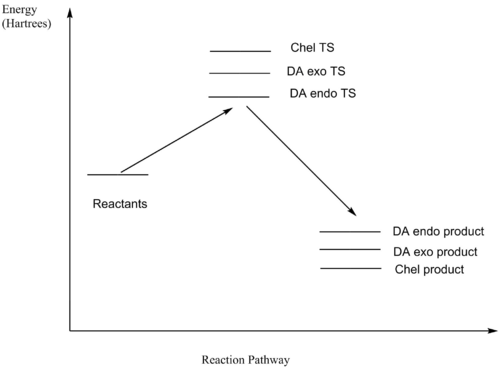
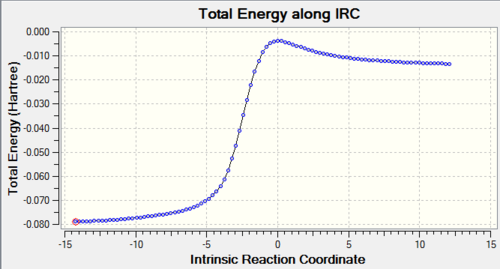
According to the data and reaction profile above, the activation energy of Cheletropic reaction is the highest, but the reaction energies of two DA reactions are higher than that of Cheletropic reaction. So under high temperature, there is sufficient energy to overcome the energy barrier, DA route is preferred because the product is thermodynamically favourable. However, under low temperature, Cheletropic route is preferred because kinetic product is preferred.
According to the IRC animations, it can be seen that a new double bond is formed in the six-membered ring to give a stable arometic benzene ring and the electrons in the six-membered ring becomes delocalised. So the overall reaction is exothermic due to the stabilisation of pi delocalised system in benzene ring.
Conclusion
In this computational lab, firstly we learned three methods of optimising minima and transition state in GaussView to locate and characterise transition structures of several pericyclic reactions, and we can decide if the DA reaction is a normal or reverse demand by comparing MOs of transition state with MOs of reactants. Furthermore, we can calculate the energy barriers and reaction energies by frequency calculations on reactants, transition states and products to judge if the reaction is thermodynamically or kinetically favourable. Besides, we can analysis the HOMO of the transition state to judge whether there is a secondary orbital interactions or steric hindrance or not. And also we can decide if the formation of bonds is synchronous or asynchronous by looking at the IRC animation.
Reference
[1]: Lide, David R. "A survey of carbon-carbon bond lengths." Tetrahedron 17.3 (1962): 125-134.
[2]: Batsanov, S. S. "Van der Waals Radii of Elements." Inorganic Materials 37.9 (2001): 871-885.
[3]:http://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bond_Distance,_Radius_and_van_der_Waals_Radius