Rep:MOD:VI4018
Molecular Modelling Lab
NH3 Molecule
Optimisation
NH Molecule |
Method: RB3LYP
Basis set: 6-31G(d,p)
Symmetry/Point group: C3v
Final energy: -56.55776873 au
RMS gradient: 0.00000485 au
Bond length: 1.01798 Å
Bond angle: 105.741°
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986273D-10 Optimization completed.
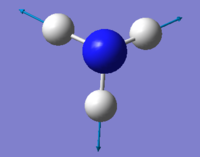
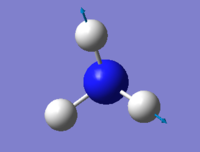
Vibrations
| wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity arbitrary units | 145 | 13.6 | 13.6 | 1.06 | 0.271 | 0.271 |
| Image | 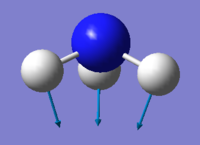 |
 |
 |
 |
 |
|
From The 3N-6 rule 6 modes are expected because there are 4 atoms in the ammonia molecule. There are 2 modes with the wavenumber of 1694 cm-1 and 2 modes with the wavenumber of 3590 cm-1. This means they are degenerate. Vibrations at 1090 and 1694 cm-1 are bending while vibrations at 3461 and 3590 cm-1 are stretching. Vibrations at 1090 and 3461 cm-1 have A1 symmetry and are highly symmetrical. Vibration at 1090 also is commonly known as the "umbrella" mode. In the IR spectrum, there are 4 peaks present, with 2 of them being clearly visible.
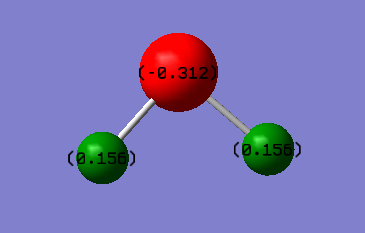
Charge
Nitrogen is more electronegative atom than hydrogen. It will have more electron density around it and therefore will be negatively charged while hydrogen atoms will be positively charged. Overall ammonia molecule is neutral so individual charges on the atoms must cancel out and they do give an overall charge of 0.
N2 Molecule
Optimisation
N Molecule |
Method: RB3LYP
Basis set: 6-31G(d,p)
Symmetry/Point group: D∞h
Final energy: -109.52412868 au
RMS gradient: 0.00000060 au
Bond length: 1.10550 Å
Bond angle: 180°
Vibrations
| Wavenumber cm-1 | 2457 |
| Symmetry | SGG |
| Intensity arbitrary units | 0 |
| Image | 
|
N2 is a linear molecule so 3N-5 expression is used to determine the number of vibrational modes available to the molecule. N2 has two atoms so it has one vibrational mode available to it. This mode is a stretching vibration which doesn't lead to the change in dipole in a molecule so it is IR inactive.
Charge
N2 consists of the atoms of the same element with the same electronegativity, therefore, there is no partial charge on any of the atoms. The overall charge of the molecule is 0.
H2 Molecule
Optimisation
H Molecule |
Method: RB3LYP
Basis set: 6-31G(d,p)
Symmetry/Point group: D∞h
Final energy: -1.17853936 au
RMS gradient: 0.00000017 au
Bond length: 0.74279 Å
Bond angle: 180°
Vibrations
| Wavenumber cm-1 | 4466 |
| Symmetry | SGG |
| Intensity arbitrary units | 0 |
| Image | 
|
H2 is a linear molecule so 3N-5 expression is used to determine the number of vibrational modes available to the molecule. H2 has two atoms so the number of vibrational modes is 1. It is a stretching vibration which doesn't lead to the change in the dipole mode so it is IR inactive.
Charge
H2 consists of the same atoms with the same electronegativity so there are no partial charges on any of the atoms. The overall charge of the molecule is 0.
Transition Metal Complex
Structure
Unique Identifier: VEJSOV
Link: https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=VEJSOV&DatabaseToSearch=Published
Bond length between Nitrogens
Bond length: 1.1165 Å
Bond length between nitrogens in the transition metal complex is longer than in the nitrogen molecule because one of the nitrogens donates the lone pair to the transition metal. This makes it positively charged and the electrons in the triple bond move towards it. This decreases the electron density between the nitrogens and therefore lowers the length of the bond.
Energy of Haber-Bosch process
Calculation
N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 au
2*E(NH3)= -113.11553746 au
E(N2)= -109.52412868 au
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 au
1 kJ/mol = 0.00038 au
Therefore ΔE = -146.5 kJ/mol
The negative sign indicates that the reaction is exothermic and that the product is more stable than the reactants.
SH2 Molecule
Optimisation
SH Molecule |
Method: RB3LYP
Basis set: 6-31G(d,p)
Symmetry/Point group: C2v
Final energy: -399.39162414 au
RMS gradient: 0.00012068 au
Bond length: 1.34737 Å
Bond angle: 92.681°
Vibrations
| Wavenumber cm-1 | 1224 | 2692 | 2712 | |
| Symmetry | A1 | A1 | B2 | |
| Intensity arbitrary units | 4.92 | 6.73 | 8.62 | |
| Image |  |
 |

|
SH2 is a non-linear molecule so to predict the number of vibrational modes 3N-6. There are three atoms in the molecule so three vibrational modes are expected. As can be seen from the IR spectrum they are all IR active as they lead to a change of dipole in the molecule.
Charge
Sulphur is more electronegative than hydrogens, therefore, has a partial negative charge and hydrogens have a partial positive charge. The overall charge of the molecule is 0 and the sum partial charges of each atom adds up to 0.
Molecular Orbitals
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES - however, you used 'Molecule' as a title for all of the displayed jmols which is in fact not very informative and you should have given the name of the molecule or its formula instead.
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - however you did miss out explaining exactly why only 2 modes are seen in the vibrational section. You correctly stated that there are two sets of degenerate modes - this explains a spectrum with 4 peaks. However there are only 2 peaks visible as peaks 4, 5 and 6 are of too low an intensity to be visible and you have not explained why the intensities are low.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - Good job showing the information in a clear way. However, you stated an bond angle for H2 and N2. This is incorrect as a minimum of 3 atoms is needed to define a bond angle!
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
You have done a good job of presenting this information, well done! Recognise there is only ONE HOMO for a given molecule, so the use of 'a HOMO of the hydrogen sulfide' is essentially not properly describing the situation.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
NO - there are no additional information indicating a deeper analysis of the data or additional calculations.