Rep:MOD:SY3814
Introduction
Potential Energy Surface
A potential energy surface(PES) is a mathematical function that describes molecule as a function of its geometry. It is commonly applied in Chemistry to theoretically explore properties of atomic structures and rates of chemical reactions.
stationary points
There are two stationary points(points with zero gradient) in PES, minimum state point and transition state point. Minimum state is where the molecule in its minimum energy shape(physically stable) and the transition state is the highest energy point along a reaction coordinate.
gradient and the curvature
As the point is evaluated in the PES, the point can be identified by its gradient(first derivatives) and curvature(second derivatives) of the energy related to the position.
Gradient
For a given molecule, r describes the position of molecule and the Function E(r) can be introduced as the energy of the position. The first derivative of the energy corresponds to the position of the molecule is calculated as gradient.
Gradient = ∂E/∂r
Curvature
In the case that the gradient is a zero vector(stationary points), second derivative matrix is used, which describes the PES curvature at r position.
Curvature = ∂∂E/∂ri∂rj
Nf710 (talk) 20:20, 20 January 2017 (UTC) This is too brief you havent defined a TS properly
Exercise 1
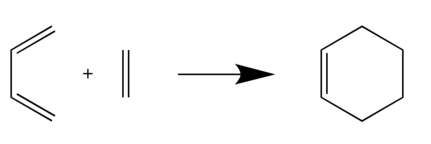
Reaction of Butadiene with Ethylene
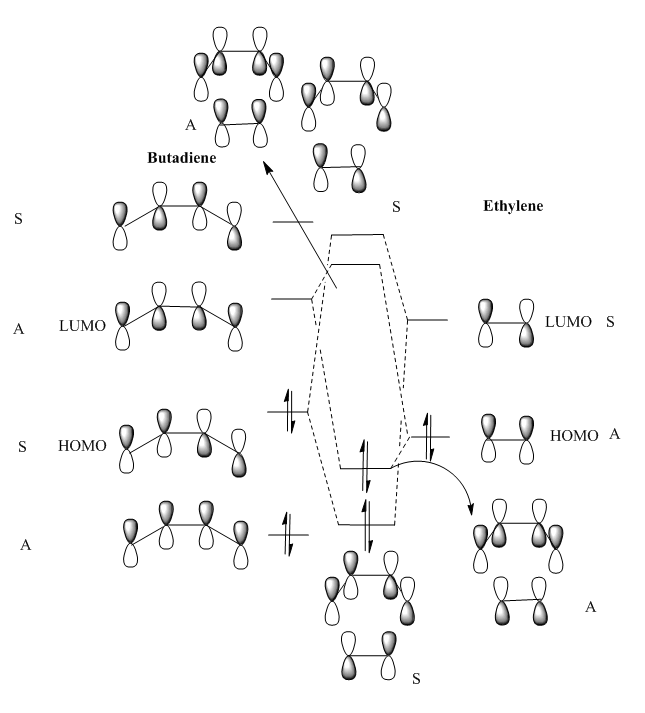
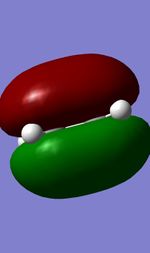
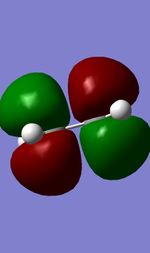
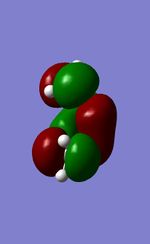
Visualised MOs
| Ethylene HOMO(A) | Ethylene LUMO(S) | Butadiene HOMO(S) | Butadiene LUMO(A) |
|---|---|---|---|
 |
 |
 |
|
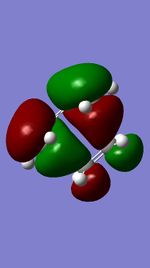
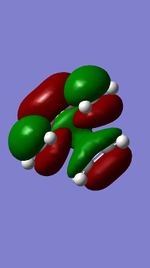
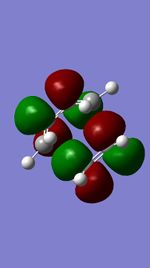
| Occupied orbital(S) | TS HOMO(A) | TS LUMO(A) | Unoccupied orbital(S) |
|---|---|---|---|
 |
 |
 |
|
MO diagram for the formation of the butadiene/ethene Transition state
The Mo diagram demonstrate that 4 TS orbital are produced by interaction between LUMO and HOMO orbitals. Correlated with Mos computed, only butadiene HOMO orbital interacts with ethylene LUMO orbital and butadiene LUMO orbital interacts with HOMO orbital in ethylene. It testifies that the reaction is allowed only when reactants in the same symmetry. The orbital overlap integral is zero in an asymmetric-asymmetric interaction and non-zero in interaction with same symmetries.
bond length change in the reaction
| Reactants | Transition state | Product | |
|---|---|---|---|
| Ethylene C=C | 1.327Å | 1.381Å | 1.54Å |
| Butadiene C-C | 1.33Å | 1.38Å | 1.50Å |
| Butadiene C=C | 1.47Å | 1.41Å | 1.34Å |
| Butadiene C-C | 1.33Å | 1.38Å | 1.50Å |
| New forming C-C bond | 2.11Å | 1.54Å | |
| New forming C-C bond | 2.11Å | 1.54Å |
The ethylene C=C double bond length is 1.327Å and the C=C bond lengths in Butadiene are at 1.33Å, 1.47Å and 1.33Å respectively. In Transition state, the C-C bond length in ethylene is extended to 1.381Å, the C-C bond lengths in butadiene are shifted to 1.38Å, 1.41Å and 1.38Å. The C-C bond length between reactants is around 2.11Å. In the product, the reactants C-C bond length are changed to 1.54Å (Ethylene C-C), 1.50Å, 1.34Å and 1.50Å (Butadiene C-C). The C-C bond length between reactants is shortened to 1.54Å. As the data shown, the C-C bond length between reactants shorten as the reaction progresses. The butadiene C-C single bond length decreases and turns into double bond in the reaction. All reactants double bonds, in contrast, move longer and change to single bonds in the product.
Vibrations
In the TS vibration, the two reacting carbons from Ethylene and Butadiene vibrate vertically and move aproaching each other. The formation of the two bonds are synchronous, and the transition movement can be testified by the negative frequency (negative second derivative for the saddle point). From the computed MOs, the vibration for the lowest positive frequency is twisting around the reaction center.
Nf710 (talk) 20:22, 20 January 2017 (UTC) This section is quite brief perhaps you ran out of time. you havent shown much understanding such as if teh reaction in inverse or normal
Exercise 2
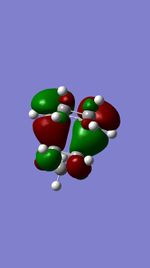
| Occupied orbital(A) | TS HOMO(S) | TS LUMO(S) | Unoccupied orbital(A) |
|---|---|---|---|
 |
 |
 |

|
| Occupied orbital(A) | TS HOMO(S) | TS LUMO(S) | Unoccupied orbital(A) |
|---|---|---|---|
 |
 |
 |
|
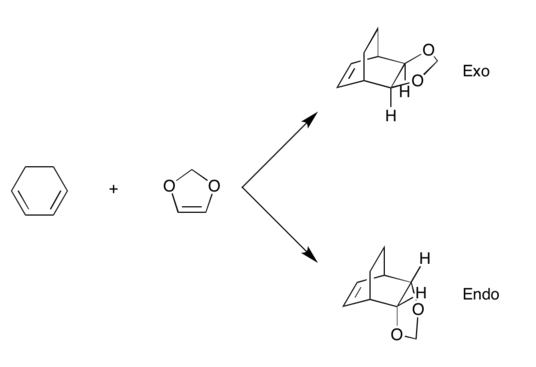
(These are orbitals for the exo product, not TS. Even so, the symmetry tags are incorrect for the MOs you've shown Tam10 (talk) 11:32, 5 January 2017 (UTC))
It's a inverse electron demand DA reaction due to the electron donating oxygen subsituents on dienophile. The electron richer dienophile makes the diene LUMO and dienophile HOMO more closer in energy and be the frontier orbitals.
Reaction Barriers and Reaction Energies
Barrier energy = transition state free energy- sum of reactant free energies Reaction free energy = sum of product free energies - sum of reactant free energies
Extract the "Sum of electronic and thermal Free Energies" data from the Log file and substitute into the equation.
Endo reaction free energy:ΔrG0(298K)=(-500.418694-(-267.068643+-233.324375))*627.5095= -16.11Kcal = -67.404KJ/mol
Exo reaction free energy:ΔrG0(298K)=(-500.417317-(-267.068643+-233.324375))*627.5095= -15.25Kcal = -63.806KJ/mol
Endo reaction barrier energy:ΔrG0(298K)=(-500.073175-(-267.068643+-233.324375))*627.5095= 17.51Kcal = 73.262KJ/mol
Exo reaction barrier energy:ΔrG0(298K)=(-500.072282-(-267.068643+-233.324375))*627.5095= 18.07Kcal = 75.605KJ/mol
Endo is the more kinetic product due to the relative lower barrier energy, and the higher reaction free energy makes the exo product more thermodynamic favoured.
Secondary orbitals and steric effects
The secondary orbital interaction (non-bonding) in endo structure makes it with lower energy and react faster towards endo product. This can be testified by the oxygen-carbon overlap in the HOMO transition state MO. The oxygen in 1,3-Dioxole interacts with the carbon in cyclohexadiene so that to lower the energy barrier and make the reaction proceed faster. The smaller crossing between two rings in exo structure makes it less steric hindered thermodynamically stable.
Nf710 (talk) 20:24, 20 January 2017 (UTC) You coudl have shown the SOO with a diagram and also your energies are incorrect and therefore you have got the wring conclusion
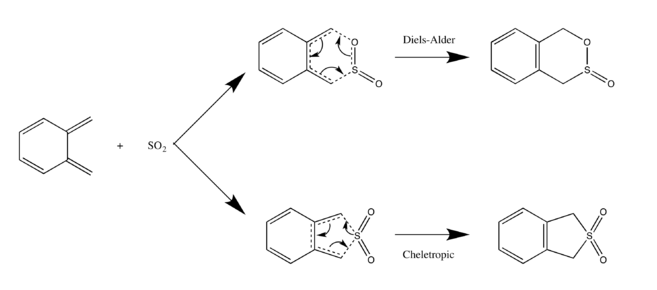
Exercise 3
| Exo- Diels-Alder Reaction | Endo- Diels-Alder Reaction | Cheletropic Reaction |
|---|---|---|
 |
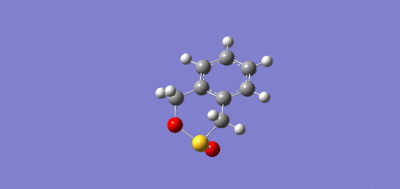 |

|
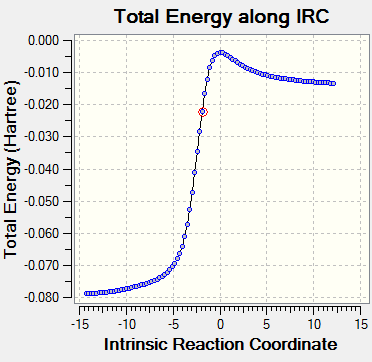 |
 |
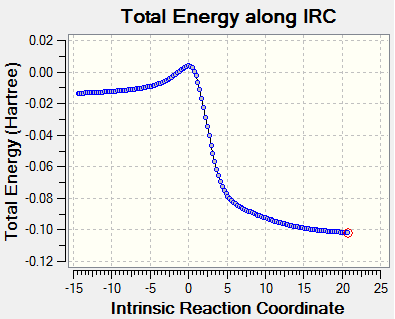
|
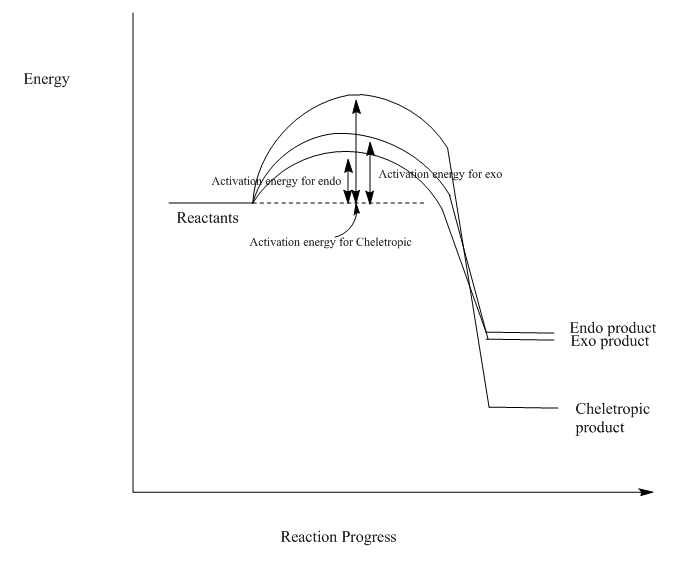
The IRC reaction paths move from product to reactants in exo and endo reaction diagrams, while for Cheletropic, it's from reactants to product.
Activation and Reaction Energies for Each Route
Endo reaction free energy:ΔrG0(298K)=(0.021699-(-0.113379+0.178117))*627.5095= -27.01Kcal = -112.999KJ/mol
Exo reaction free energy:ΔrG0(298K)=(0.021455-(-0.113379+0.178117))*627.5095= -27.16Kcal = -113.637KJ/mol
Cheletropic reaction free energy:ΔrG0(298K)=(-0.000002-(-0.113379+0.178117))*627.5095= -40.63Kcal = -169.996KJ/mol
Endo reaction activation energy:ΔrG0(298K)=(0.090560-(-0.113379+0.178117))*627.5095= 16.20Kcal = 67.796KJ/mol
Exo reaction activation energy:ΔrG0(298K)=(0.092076-(-0.113379+0.178117))*627.5095= 17.15Kcal = 71.756KJ/mol
Cheletropic activation free energy:ΔrG0(298K)=(0.099061-(-0.113379+0.178117))*627.5095= 21.53Kcal = 90.082KJ/mol
(This data should be tabulated for ease of reading Tam10 (talk) 11:32, 5 January 2017 (UTC))
From the calculation, the endo route is the most preferred one due to its lowest activation energy, which makes the reaction progresses faster towards endo product(kinetic product). However, as the Diels-Alder reaction move towards the equilibrium, more relatively thermodynamic exo product will be formed. The Cheletropic product has the lowest energy level, so it's most thermodynamically favoured and can be formed in the reversible reaction.
Reaction Profile
(Use straight lines between R-TS-P Tam10 (talk) 11:32, 5 January 2017 (UTC))
Xylylene bonding change in the reaction
From the IRC animation, the C-C single bonds in 6 membered ring shorten and with more double bond character as the reaction progresses. The C=C double bond length moves longer and shares the π character across the ring.
Conclusion
Transition states of three Diels-Alder reaction have been investigated in this experiment, the bond length changes and free energy level are clearly shown in the Gaussview files. From the computed MOs and data, the endo adduct is usually the kinetic product, which is influenced by secondary orbital interaction to lower its barrier energy. While the less steric congestion makes the exo adduct more thermodynamic favourable and will be the final product in the equilibrium.