Rep:MOD:ST3515 TS3
Introduction
A potential energy surface describes the relationship between the energy of a molecule and its geometry. The transition state (TS) is the local maximum on a potential energy surface while a minimum is the local minimum on a potential energy surface, corresponding to either reactants or products. Both maximum and minimum have the same first derivative of energy ie. zero gradient, but their second derivatives are different. A maximum has a negative second derivative while a minimum has a positive second derivative.
Computational methods can be used to distinguish a transition state from a minimum. A frequency analysis will show that the transition state has only one negative frequency but a minimum will not have any negative frequencies. Computational methods also allow these transitions states that cannot be isolated to be visualised. The computed energies of the TS, reactants and products also allow reaction energies and reaction barriers to be calculated, and hence predict the more favourable reaction pathway. Once the TS has been optimised successfully, an intrinsic reaction coordinate (IRC) calculation can be carried out which allows visualisation of the minimum energy pathway from reactants to products.
Nf710 (talk) 19:31, 5 March 2018 (UTC)You have only talked about the PES in 1 dimension it is 3N-6 dimensions. Furthermore you get the force constants by diagonalising the hessian. this allows you to distinguish between a TS and a minimum in higher dimensions.
The semi-empirical method PM6 involves approximations and the use of atomic and diatomic parameters from experimental data. Calculations using this method are therefore fast and are frequently used to minimise structures before carrying out more expensive and time consuming calculations. B3LYP calculations, a Density Functional Theory (DFT) method, uses a greater number of basis functions than PM6. Hence calculations using this method take longer but are more accurate than PM6.
Nf710 (talk) 19:31, 5 March 2018 (UTC) Correct about the method however DFT can use a variable set of basis functions (you choose) and PM6 has a built in basis set.
The cycloaddition reactions of butadiene with ethene, cyclohexadiene with 1,3-dioxole and o-xylylene with SO2 were studied using the PM6 method. The cyclohexadiene/1,3-dioxole system was also further optimised using B3LYP using the basis set 6-31G(d) (this basis set has added d polarisation functions on atoms other than hydrogen). The following procedure was carried out for all three systems: firstly, the products of the reactions were optimised to a minimum and the resulting optimised structures were modified to resemble the TS. The modified structure was then optimised to a TS. Reactants were built and optimised to a minimum.
Exercise 1: Butadiene and Ethene
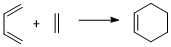
Butadiene and ethene undergo a [4+2] Diels Alder reaction (Scheme 1). The semi-empirical PM6 method was used to optimise the reactants, product and TS. Frequency analysis suggests successful optimisation of the structures, with the TS structure having one imaginary frequency at -948.72 cm-1 and the reactants and product having no imaginary frequencies.
MO Analysis
| MO | Butadiene | Butadiene/Ethene TS | Ethene | ||||||
| LUMO + 1 | |||||||||
| LUMO | |||||||||
| HOMO | |||||||||
| HOMO - 1 |
(Fv611 (talk) You should have added labels to your MOs both in the JMol table and in the diagram. Additionally, the change in energy levels is not due to mixing, but to this being a TS MO diagram rather than a product one.)

The π MOs of butadiene and ethene (HOMOs and LUMOs) involved in the formation of the TS were computed. The four TS MOs resulting from the interaction of the HOMO and LUMO of the reactants were also computed (Table 1).
An allowed reaction is one in which the interacting MOs, which are close enough in energy for effective orbital overlap, are of the same symmetry. Hence symmetric-symmetric MO interactions and asymmetric-asymmetric MO interactions are allowed. For these allowed reactions, the orbital overlap integral is non-zero. The orbital overlap integral is zero when there is a symmetric-asymmetric MO interaction, and this corresponds to a forbidden reaction.
The computed MOs and MO diagram for the Diels Alder reaction between butadiene and ethene show that the MO interactions are allowed (Table 1; Figure 1). The asymmetric HOMO of butadiene interacts with the asymmetric LUMO of ethene to produce two asymmetric TS MOs. The symmetric LUMO of butadiene interacts with the symmetric HOMO of ethene to produce two symmetric TS MOs.
The TS MO energies computed however show the bonding TS MOs to be higher in energy and the antibonding MOs to be lower in energy than expected (Figure 1). This suggests that there is MO mixing in the TS causing a shift in the MO energies.
Bond Length Analysis
| C-C single bond length1 | C=C double bond length1 | Carbon Van der Waals radius2 |
|---|---|---|
| 1.54 | 1.34 | 1.85 |
In the TS, the bond lengths are between the literature values for the C-C single bond length and C=C double bond length, suggesting a change in bond order of the bonds is occurring during the reaction. As the reaction progresses, the bond lengths C1, C3 and C5 increase from ~1.3 Å in the reactants to ~1.5 Å in the product. This suggests that the double bonds at those positions are breaking to form a single bond. The bond length C2 decreases from 1.47 Å in the reactants to 1.34 Å in the product corresponding to formation of a double bond from a single bond at that position. (Tables 2 and 3).
In the TS, the bond lengths C4 and C6 are less than twice the Van der Waals radius of carbon, suggesting that bond formation is occurring at those positions. In the product, these bond lengths are the same as the lit. C-C single bond length of 1.54 Å, suggesting that single bonds were formed at those positions (Tables 2 and 3).
TS Vibrational Analysis
Figure 2. Vibration corresponding to the reaction at the TS. |
Frequency analysis of the TS shows one imaginary frequency at -948.72 cm-1, suggesting that the TS structure was correctly optimised. This imaginary frequency corresponds to the reaction at the TS and shows synchronous bond formation with both bonds forming to the same extent (Figure 2). This is in agreement with the concerted mechanism of a Diels Alder reaction.
Exercise 2: Cyclohexadiene and 1,3-Dioxole
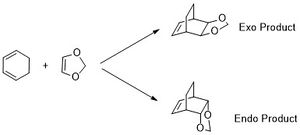
Cyclohexadiene and 1,3-dioxole can undergo a [4+2] Diels Alder reaction to form either the exo or endo product (Scheme 2). The semi-empirical PM6 method was used to obtain initial structures of the reactants, products and TS. These initial structures were then optimised using B3LYP method using the basis set 6-31G(d). Frequency analysis suggests that the TS, reactant and product structures were optimised correctly, with the reactants and products having no imaginary frequencies and the endo and exo TS having one imaginary frequency at -520.96 cm-1 and -528.82 cm-1 respectively.
MO Analysis
| MO | Cyclohexadiene | 1,3-Dioxole | ||||
| LUMO | ||||||
| HOMO |
| MO | Endo TS | Exo TS |
| LUMO + 1 | 
|
|
| LUMO | 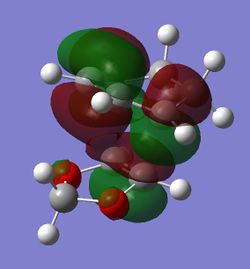
|
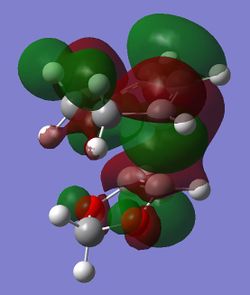
|
| HOMO | 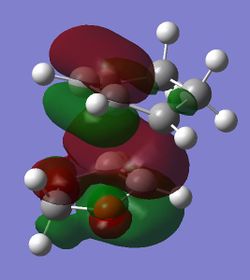
|

|
| HOMO - 1 | 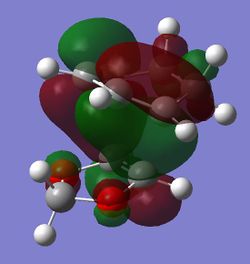
|

|

(Fv611 (talk) Your MO diagram does not reflect the energies you should have computed for the TS. Also you do not discuss the difference between the related MO energies in the exo and endo conformation)
The computed MOs and MO diagram for the Diels Alder reaction between cyclohexadiene and 1,3-dioxole show that the MO interactions are allowed (Tables 4 and 5; Figure 3).
The normal demand Diels Alder reaction involves an electron poor dienophile and an electron rich diene. However the Diels Alder reaction can also occur between an electron rich dienophile and an electron poor diene, and this is known as the inverse demand Diels Alder reaction. In such a reaction, the HOMO and LUMO energy is raised for an electron rich dienophile, meaning that the strongest interaction is between the HOMO and LUMO of the dienophile and diene respectively, rather than between the HOMO and LUMO of the diene and dienophile respectively. The opposite is true for a normal demand Diels Alder reaction.
1,3-dioxole is an electron rich dienophile, while cyclohexadiene is an electron poor diene. The oxygen atoms in 1,3-dioxole are electron donating and hence raise the energy of its HOMO and LUMO. This makes the interaction between the HOMO of 1,3-dioxole and the LUMO of cyclohexadiene stronger than the interaction between the LUMO of dioxole and the HOMO of cyclohexadiene (Figure 3). Hence this is an inverse demand Diels Alder reaction.
Nf710 (talk) 19:33, 5 March 2018 (UTC) How do you know this? You can test it by dong a single point energy of the reactant geoms on the IRC.
Reaction Energies and Reaction Barriers
| Adduct | Reactants | TS | Product | Reaction Energy | Reaction Barrier |
|---|---|---|---|---|---|
| Endo | -1313781 | -1313621 | -1313849 | -67.410 | 159.809 |
| Exo | -1313781 | -1313614 | -1313845 | -63.808 | 167.649 |
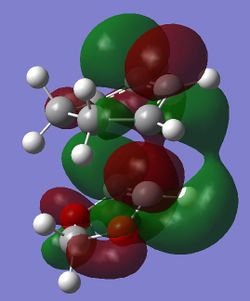
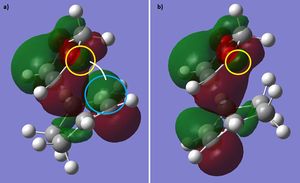
The HOMO of the endo TS suggests that there is a favourable secondary orbital interaction between the orbitals on oxygen in 1,3-dioxole and the orbitals on cyclohexadiene. This interaction is not possible in the HOMO of the exo TS as the orbitals involved cannot overlap (Figure 4). The steric hindrance between the reactants, which would increase the TS energy, is similar in both endo and exo TS (Figure 4). Therefore the endo TS is lower in energy than the exo TS due to the presence of the secondary orbital interaction in the endo TS. This makes the endo product the kinetic product as it has a lower reaction barrier, in agreement with the calculated reaction barriers (Table 6.). However, the exo product is lower in energy than the endo product, making the exo product thermodynamically favourable (Table 6.).
Nf710 (talk) 19:37, 5 March 2018 (UTC) your energies are correct and you have shown the SOO quite nicely. Why is the exo the thermo product? Also this section is quite brief and you could have gone into alot more detail in places.
Exercise 3: SO2 and o-Xylylene
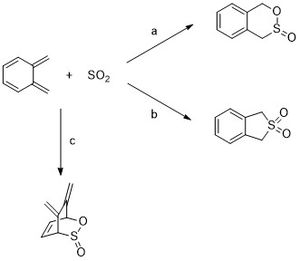
SO2 and o-xylylene can undergo Diels Alder reaction to form the exo or endo product and also a cheletropic reaction to form a five membered ring (Scheme 3a and 3b). The semi-empirical PM6 method was used to optimise the reactants, products and TS. Frequency analysis suggests that the reactants, products and TS were optimised successfully. The endo and exo TS structures showed one imaginary frequency at -333.75 cm-1 and -351.49 cm-1 respectively. The cheletropic TS showed one imaginary frequency at -121.34 cm-1. The reactant and product structures showed no imaginary frequencies.
The IRCs for each path confirm that the reactions are either Diel Alder or cheletropic. It also shows how an aromatic benzene ring forms as a result of the reactions (Table 7). This explains the high instability of the non-aromatic o-xylylene that wants to react to form the thermodynamically stable aromatic benzene ring. Besides reaction with another molecule, o-xylylene can also undergo an electrocyclic reaction to form the stable aromatic ring (Scheme 4).
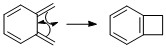
(Xylyene can indeed rearrange into this aromatic product, but you would need to compute the barrier to determine if this is a significant side-product Tam10 (talk) 11:59, 2 March 2018 (UTC))
Reaction Energies and Reaction Barriers

(Putting the reaction energies and barriers on this diagram would make comparison easier Tam10 (talk) 11:59, 2 March 2018 (UTC))
| Reactants | TS | Product | Reaction Energy | Reaction Barrier | |
|---|---|---|---|---|---|
| Endo Diels Alder | 154.351 | 237.765 | 56.989 | -97.361 | 83.415 |
| Exo Diels Alder | 154.351 | 241.748 | 56.325 | -98.026 | 87.398 |
| Cheletropic | 154.351 | 260.084 | 0.013 | -154.337 | 105.734 |
(Your reactant energies are a bit off but as you've included the files I can see that it converged to the correct criteria Tam10 (talk) 11:59, 2 March 2018 (UTC))
The endo product of the Diels Alder reaction is the kinetic product with the lowest activation energy. The exo product of the Diels Alder reaction is more stable than the endo product as it has a larger reaction energy. The cheletropic product is the thermodyanamic product as it has the most negative reaction energy. However it also has the highest activation energy (Figure 5; Table 8 ).
Reaction with Second cis-Butadiene Fragment
| Adduct | Reactants | TS | Products | Reaction Energy | Reaction Barrier |
|---|---|---|---|---|---|
| Endo | 154.351 | 267.985 | 172.260 | 17.909 | 113.634 |
| Exo | 154.351 | 275.822 | 176.710 | 22.359 | 121.471 |
SO2 can also undergo Diels Alder reaction with a second cis-butadiene fragment in the six-membered ring of o-xylylene (Scheme 3c). This reaction is however thermodynamically unfavourable because the resulting products do not contain the aromatic benzene ring and hence are less stable. This is seen in the computed reaction energies that are much lower than the reaction energies involving reaction with the first cis-butadiene fragment. Furthermore, the positive reaction energies show that the reactions are endothermic (Table 9).
The reaction is also not kinetically favourable because the reaction barriers are higher in energy than those for the Diels Alder reactions involving the first cis-butadiene fragment and the Cheletropic reaction (Table 9).
Conclusion
The reactants, products and TS of the three systems were successfully optimised using PM6 method. For the cyclohexadiene/1,3-dioxole system, B3LYP was also used to optimise the structures successfully. Frequency analysis showed that all TS have one imaginary frequency and the reactants and products have no imaginary frequencies.
For the Diels Alder reaction between butadiene and ethene, the visualised MOs were used to plot the MO diagram and showed that only MOs of the same symmetry can interact to produce new MOs. Bond lengths of the reactants, TS and product were used to study how the bond order changes as the reaction progressed. The imaginary frequency of the TS was also visualised and showed that the bond forming step is synchronous.
Visualised MOs were used to construct the MO diagram for the Diels Alder reaction between cyclohexadiene and 1,3-dioxole and showed that this is an inverse demand Diels Alder reaction. The energies of the structures were used to calculate the reaction energies and reaction barriers for both endo and exo pathways. The endo pathway was calculated to have the lowest reaction barrier, due to favourable secondary orbital interactions, and hence the endo product is the kinetic product. The exo pathway has the most negative reaction energy and hence the exo product is the thermodynamic product.
For the reaction between o-xylylene and SO2, the reaction energies and reaction barriers calculated suggest that the endo Diels Alder pathway has the lowest reaction barrier and the cheletropic pathway has the most negative reaction energy. Hence the endo product and the cheletropic product are the kinetic and thermodynamic products respectively. Diels Alder reaction between SO2 and a second cis-butadiene fragment in o-xylylene was also shown to be kinetically and thermodynamically unfavourable, as both endo and exo pathways have positive reaction energies and a much higher reaction barrier than the pathways involving the first cis-butadiene fragment.
References
1. J. E. Proctor, D. A. M. Armada, A. Vijayaraghavan, An Introduction to Graphene and Carbon Nanotubes, CRC Press, 2017.
2. S. S. Batsanov, Inorg. Mater., 2001, 37, 878.