Rep:MOD:SG3415
Gaussian has been used to optimise and analyse NH3, N2 and H2 then calculate the energies for the Haber Process to establish that ammonia is more stable than the reactant gases. ClF was also examined in the same way.
NH3 molecule
Summary
Molecule name: NH3, ammonia
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy E(RB3LYP): -56.55776873 a.u.
RMS gradient: 0.00000485 a.u.
Point group: C3V
N-H bond length: 1.01798 angstrom*
N-H bond angle: 105.741 degrees
* A literature value for the N-H bond length is 1.019 +/-0.002 angstrom. The value calculated by Gaussian was within the uncertainty limit of the literature value. The same source gave a literature value for the H-N-H bond angle as 109.1 +/- 1 degree. The calculated values are not exactly the same because Gaussian has a small iterative process (when compared to a more complex program), meaning that the value is fairly accurate but not precise past two decimal places.
*Reference: J. P. Guillor, L. S. Bartell, The Journal of Chemical Physics, 1968, 49(6), 2488-2493.
Item table from *.log file
Item Value Threshold Converged
Force 0.000004 0.000450 YES
Force 0.000004 0.000300 YES
Displacement 0.000072 0.001800 YES
Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986286D-10
Optimization completed.
-- Stationary point found.
Dynamic image of ammonia
Ammonia |
The optimisation file is linked to here
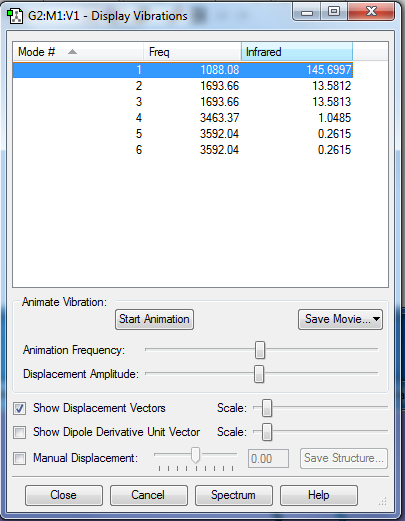
Screenshot to show the vibrations of an NH3 molecule
Display vibrations questions
1. How many modes do you expect from the 3N-6 rule? N=4, therefore expect 6 modes 2. Which modes are degenerate? 2&3 and 5&6 3. Which modes are “bending” vibrations and which are “bond stretching” vibrations? 1, 2 and 3 are “bending”. 4, 5 and 6 are “stretching” 4. Which mode is highly symmetric? 4 5. One mode is known as the “umbrella” mode, which one is it? 1 6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2. As two of the modes are degenerate with others, there could be 4 bands on an experimental spectrum. However, the intensity of the peaks for 5&6 is so small that they would not be observed compared to the intensity of the other peaks.
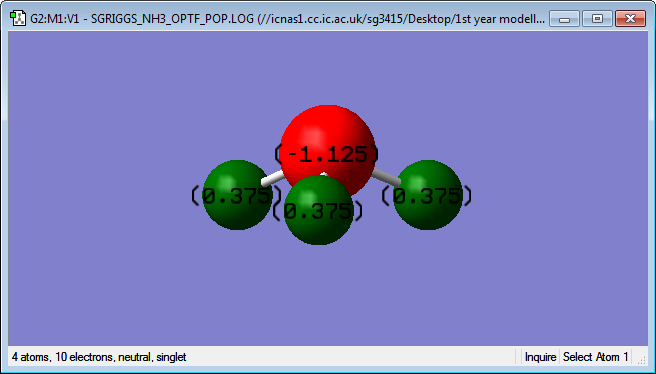
Charges on atoms in an ammonia molecule
As can be seen from the image above, N has a charge of -1.125, while H has a charge of +0.375.
The charge on N is expected to be negative because it is much more electronegative than H. This means it “pulls” the electrons closer to itself, making the N-H bond have some ionic character. N has more protons than H but the same quantum number so electrons surrounding it feel a stronger effective nuclear charge.
N2
Summary
Molecule name: N2, nitrogen
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy E(RB3LYP): -109.52412867 a.u.
RMS gradient: 0.00004425 a.u.
Point group: D*H
N-N bond length: 1.10552 angstrom
N-N bond angle: N/A as three points are required for an angle. N2 is a linear molecule
Item table from *.log file
Item Value Threshold Converged?
Maximum Force 0.000077 0.000450 YES
RMS Force 0.000077 0.000300 YES
Maximum Displacement 0.000024 0.001800 YES
RMS Displacement 0.000034 0.001200 YES
Predicted change in Energy=-1.836250D-09
Optimization completed.
-- Stationary point found.
Dynamic image of nitrogen
Nitrogen |
The optimisation file is linked to here
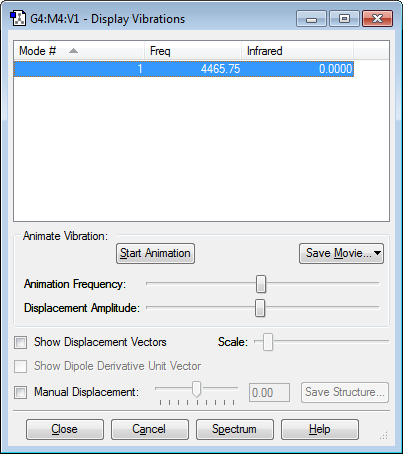
Screenshot to show the vibrations of an N2 molecule
Display vibrations questions
1. How many modes do you expect from the 3N-5 rule? N=2, therefore expect 1 mode 2. Which modes are degenerate? N/A 3. Which modes are “bending” vibrations and which are “bond stretching” vibrations? This mode is stretching 4. Which mode is highly symmetric? 1 5. One mode is known as the “umbrella” mode, which one is it? N/A 6. How many bands would you expect to see in an experimental spectrum of gaseous nitrogen? 0. The infrared value is given as zero because the vibration is symmetrical so there is no change in dipole moment.
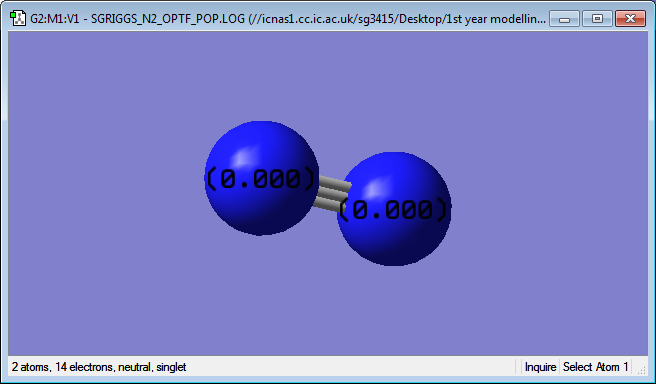
Charges on atoms in a nitrogen molecule
As can be seen, the charge on each N atom is 0, as expected. This is expected because there is no electronegativity difference between the two atoms as they are the same. This means the average electron distribution will be equal between the two atoms.
H2
Summary
Molecule name: H2, hydrogen
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy E(RB3LYP): -1.17853936 a.u.
RMS gradient: 0.00000204 a.u.
Point group: D*H
H-H bond length: 0.74297 angstrom
H-H bond angle: N/A as three points are required for an angle. H2 is a linear molecule
Item table from *.log file
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000005 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Predicted change in Energy=-1.634290D-11
Optimization completed.
-- Stationary point found.
Dynamic image of hydrogen
Hydrogen |
The optimisation file is linked to here
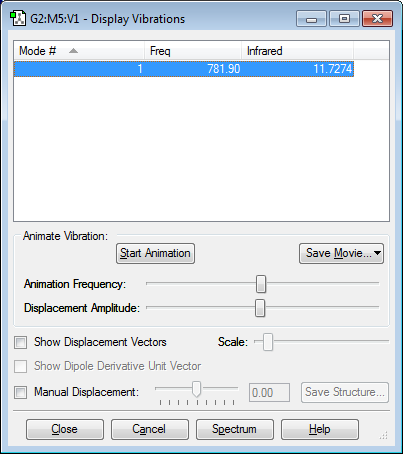
Screenshot to show the vibrations of an H2 molecule
Display vibrations questions
1. How many modes do you expect from the 3N-5 rule? N=2, therefore expect 1 mode 2. Which modes are degenerate? N/A 3. Which modes are “bending” vibrations and which are “bond stretching” vibrations? This mode is stretching 4. Which mode is highly symmetric? 1 5. One mode is known as the “umbrella” mode, which one is it? N/A 6. How many bands would you expect to see in an experimental spectrum of gaseous hydrogen? 0. The infrared value is given as zero because the vibration is symmetrical so there is no change in dipole moment.
Charges on atoms in a nitrogen molecule
As can be seen, the charge on each H atom is 0, as expected. This is expected because there is no electronegativity difference between the two atoms as they are the same. This means the average electron distribution will be equal between the two atoms.
Reactivity of ammonia
E(NH3)= -56.55776873 a.u. 2*E(NH3)= -113.1155375 a.u. E(N2)= -109.52412867 a.u E(H2)= -1.17853936 a.u. 3*E(H2)= -3.53561808 a.u. ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579075 a.u. ΔE= -146.4786141 kJ/mol = -146.48 kJ/mol
Ammonia is more stable than the reactant molecules because -146.47 kJ/mol energy is released upon formation of ammonia from the reactants. This means the product is lower in energy and so more stable.
ClF molecule
Summary
Molecule name: ClF
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy E(RB3LYP): -559.94269589 a.u.
RMS gradient: 0.00000890 a.u.
Point group: C*V
Cl-F bond length: 1.66391 angstrom
Cl-F bond angle: N/A as three points are required for an angle. ClF is a linear molecule
Item table from *.log file
Item Value Threshold Converged?
Maximum Force 0.000015 0.000450 YES
RMS Force 0.000015 0.000300 YES
Maximum Displacement 0.000027 0.001800 YES
RMS Displacement 0.000038 0.001200 YES
Predicted change in Energy=-4.174415D-10
Optimization completed.
-- Stationary point found.
Dynamic image of ClF
ClF |
The optimisation file is linked to here
Screenshot to show the vibrations of a ClF molecule
Display vibrations questions
1. How many modes do you expect from the 3N-5 rule? N=2, therefore expect 1 mode 2. Which modes are degenerate? N/A 3. Which modes are “bending” vibrations and which are “bond stretching” vibrations? This mode is stretching 4. Which mode is highly symmetric? 1 5. One mode is known as the “umbrella” mode, which one is it? N/A 6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 0. The vibration is symmetrical so there is no change in dipole moment, meaning there is no peak displayed on the IR spectrum.
Charges on atoms in a ClF molecule
As can be seen from the image above, Cl has a charge of +0.309, while F has a charge of -0.309.
This is as expected because F is more electronegative than Cl, meaning it "pulls" the electrons closer to itself. The Cl atom is less electronegative because it has an extra "layer" of electrons due to the higher quantum number, which act to shield the nuclear charge from the valence electrons, meaning electrons are not held as closely to the nucleus as in F. The values are equal and opposite because they reflect the oxidation states of the atoms in the molecule: +1 for Cl and -1 for F.
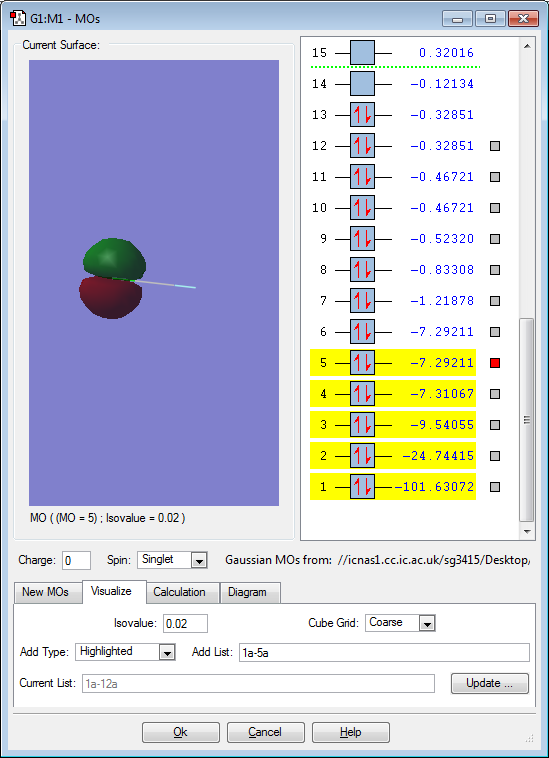
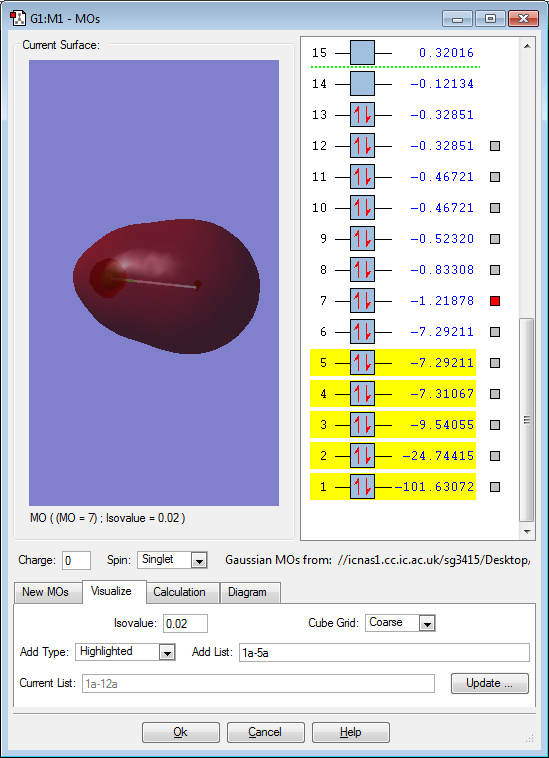
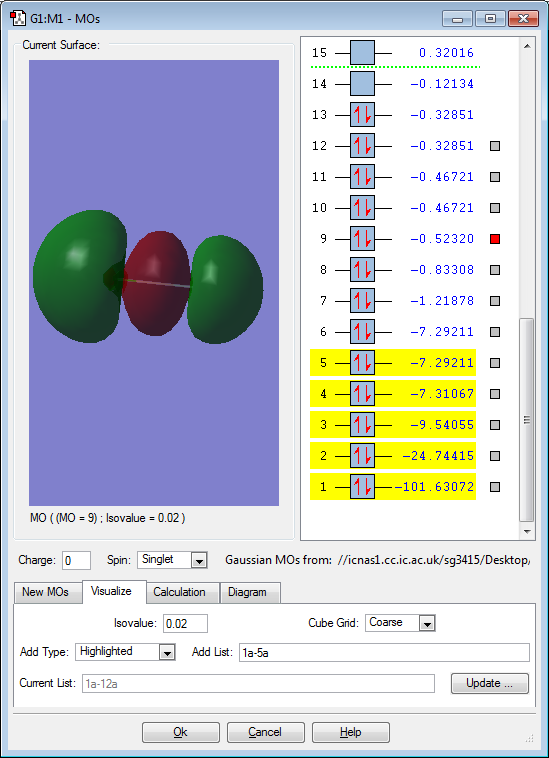
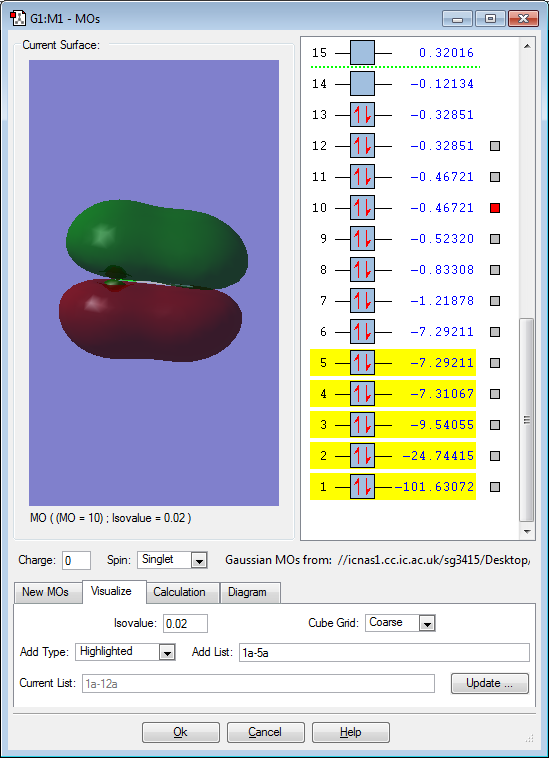
Molecular orbitals for ClF
The above image shows the filled non-bonding 1s orbital on the Cl atom. The image only has electron density around the Cl because it is of such low energy that it cannot be involved in bonding because the electrons are held very close to the Cl nucleus (making them so stable).
The image above shows one of the filled non-bonding 2p orbitals on the Cl atom. Again the image only has electron density around the Cl atom because the 2p orbital is also of an energy that is too low to contribute to a bonding molecular orbital. The orbital is two colours due to the in-phase and out-of-phase parts of the orbital. There are three non-bonding 2p Cl orbitals (all of which are filled), each orientated on a different axis (2px, 2py and 2pz).
This molecular orbital is the bonding orbital between the 2s F atomic orbital and the 3s Cl atomic orbital. Here, both the atomic orbitals are in phase (the same colour), which leads to a sigma overlap. Sigma overlap occurs along the internuclear axis, and so is symmetrical about this axis. A sigma bond is not formed on account of this overlap though because the corresponding antibonding orbital is also filled.
This image shows the filled bonding orbital formed from the overlap of the 2p F orbital and 3p Cl orbital. This is occurring through head-on overlap and so forms a sigma bond. This accounts for the single bond in the ClF molecule because the corresponding sigma antibonding orbital is not filled.
This image shows the filled pi bonding orbital formed from the overlap of the 2p F orbital and 3p Cl orbital. This is a bonding orbital because the p orbitals are in phase with each other so overlap constructively to create an area of bonding electron density above and below the sigma bond. The ClF molecule still exhibits a bond order of one however (and no pi bond) because the corresponding antibonding orbital is also filled. It can also be seen that the electrons are slightly further from the Cl nucleus than the F nucleus because the effective nuclear charge of Cl is less than that of F. This is because Cl has a higher quantum number so the electrons are more shielded in Cl and so are not held so closely to the nucleus.