Rep:MOD:OOR10969
Introduction
NH3,N2,H2 and CLF molecules were investigated by using Gaussian to find out their structural information and optimal energies. Reaction energies that calculated by using optimal energies were compared with literature values.
NH3 molecule
1. NH3 Bond Length/ Bond Angle/ Point Group
| bond length | bond angle | point group |
| 1.01798 | 105.741 | C3v |
2. Calculation Method& Basis set
| Calculation Method | Basis Set |
|---|---|
| RB3LYP | 6-31G(d,p) |
3. final energy E(RB3LYP) in atomic units (au)
-56.55776873 a.u.
4. RMS gradient
0.00000485 a.u.
5. Item
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986279D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
6. Jmol
NH3 |
The optimisation file is linkd to here
7. IR Vibrations

6 modes are expected according to 3N-6 rule. 3*4-6=6
Mode 2 and 3 are degenerate. Mode 5 and 6 are degenerate. Because they have the same frequency thus they have the same energy.
Mode 1,2 and 3 are bending vibrations. Mode 4,5 and 6 are stretching vibrations. Mode 4 is highly symmetric.
Mode 1 is the umbrella mode.
2 bands (Mode 1 and Mode 2/3) would be expected to see in an experimental spectrum as these 2 modes have large dipole moment changes and thus they have relatively high intensities on the spectrum.
8. Charge
| N charge | H charge |
|---|---|
| -1.125 | 0.0375 |
For N atom, negative charge is expected. For H atom, positive charge is expected. This is because N atom is more electronegative than H atom.
N2 molecule
1. N2 Bond Length/ Bond Angle/ Point Group
| bond length | bond angle | point group |
| 1.10550798 | linear | D*H |
2. Calculation Method& Basis set
| Calculation Method | Basis Set |
|---|---|
| RB3LYP | 6-31G(d,p) |
3. Final energy E(RB3LYP) in atomic units (au)
-109.52412868 a.u.
4. RMS gradient
0.00000060a.u.
5. Item
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.383641D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
6. IR Vibrations
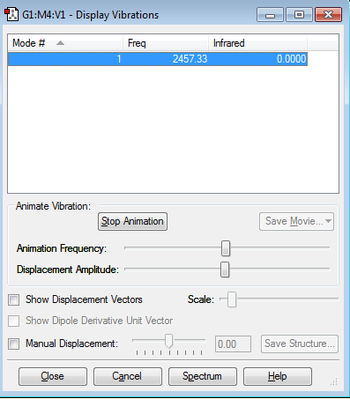
As the diatomic rule suggests 3N-5, 3*2-5=1 only one peak would be observed on the spectrum.
8. Jmol
N2 |
The optimisation file is linkd to here
9. Charge
| N charge | N charge |
|---|---|
| 0 | 0 |
Nitrogen is a homonuclear diatomic molecule therefore zero charge would be expected.
H2 molecule
1. H2 Bond Length/ Bond Angle/ Point Group
| bond length | bond angle | point group |
| 0.60000 | linear | D*H |
2. Calculation Method& Basis set
| Calculation Method | Basis Set |
|---|---|
| RB3LYP | 6-31G(d,p) |
3. final energy E(RB3LYP) in atomic units (au)
-1.17853936 a.u.
4. RMS gradient
0.00000017a.u.
5. Item
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.167772D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
-------------------------------------------------------------
6. IR Vibrations
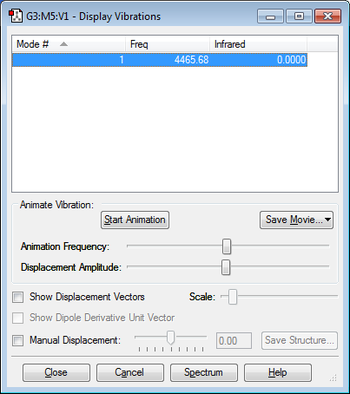
As the diatomic rule suggests 3N-5, 3*2-5=1 only one peak would be observed on the spectrum.
7. Jmol
H2 |
The optimisation file is linkd to here
8. Charge
| H charge | H charge |
|---|---|
| 0 | 0 |
Hydrogen is a homonuclear diatomic molecule therefore zero charge on hydrogen would be expected.
Reaction Energy
| E(NH3) | E(N2) | E(H2) |
| -56.55776873 a.u. | -109.52412868 a.u. | -1.17853936 a.u. |
| 2*E(NH3) | 3*E(H2) | ΔE |
| -113.1155375 a.u. | -3.5356181 a.u. | -0.11356825 a.u. |
ΔE=2*Energy(NH3)-[Energy(N2)+3*Energy(H2)]=-0.0556326 a.u.=-146.06 Kj/mol
This is the enthalpy required to make 2 mol of NH3
ammonia product is more stable than the gaseous reactants as the reaction is exothermic.
compare with literature value
The literature value of standard enthalpy of formation is -46.2KJ/mol and it differs quite a lot from the calculated value -73.03 KJ/mol [1] </references>
Project molecule--CLF
1. CLF Bond Length/ Bond Angle/ Point Group
| bond length | bond angle | point group |
| 1.66434 | linear | C*V |
2. Calculation Method& Basis set
| Calculation Method | Basis Set |
|---|---|
| RB3LYP | 6-31G(d,p) |
4. final energy E(RB3LYP) in atomic units (au)
-559.94269578 a.u.
5. RMS gradient
0.00014211 a.u.
6. Item
Item Value Threshold Converged?
Maximum Force 0.000246 0.000450 YES
RMS Force 0.000246 0.000300 YES
Maximum Displacement 0.000433 0.001800 YES
RMS Displacement 0.000613 0.001200 YES
Predicted change in Energy=-1.066054D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.6643 -DE/DX = -0.0002 !
--------------------------------------------------------------------------------
7. Jmol
CLF |
The optimisation file is linkd to here
8. IR Vibrations
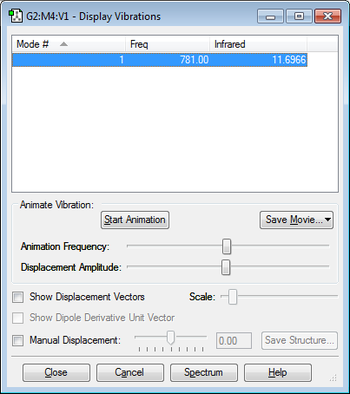
As the diatomic rule suggests 3N-5, 3*2-5=1 only one peak would be observed on the spectrum.
9. Charge
| CL charge | F charge |
|---|---|
| 0.309 | -0.309 |
The charge of florine is expected to be negative and the charge of chlorine is expected be positive as florine is more electronegative than chlorine.
Jmol of chlorine
CL2 |
The optimisation file is linkd to here
Reaction Energy
| CL2 energy | F2 energy |
|---|---|
| -920.34987886 a.u. | -199.49825218 a.u. |
ΔE=Energy(ClF)-[Energy(Cl2)+Energy(F2)]/2=-0.01863026 a.u.=-48.92 KJ/mol
This is the enthalpy required to make 1 mole of ClF
11. Compare with literature value
The literature value of standard enthalpy of formation is -56.5KJ/mol and it is relatively close to the calculated value -48.92 KJ/mol [2] </references>
Jmol of Florine
F2 |
The optimisation file is linkd to here
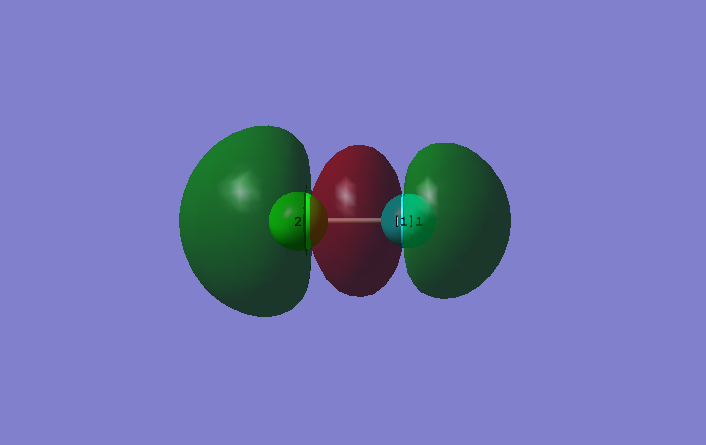
10. Molecular Orbitals
This is the antibonding combination between 3s orbital in Cl and 2s orbital in F. Molecular orbital is occupied and in the HOMO region. Cl contributes more in the antibonding orbital.This is because 3s orbital in Cl is higher in energy than 2s orbital in F therefore 3s orbital in Cl contributes more in to the antibonding orbital.
This is the bonding combination between 3s orbital in Cl and 2s orbital in F.As F is more electronegative in the occupied bonding orbital therefore it distorts more electron density towards itself.
This is an occupied bonding combination between 3pZ orbital in Cl and 2pZ orbital in F.
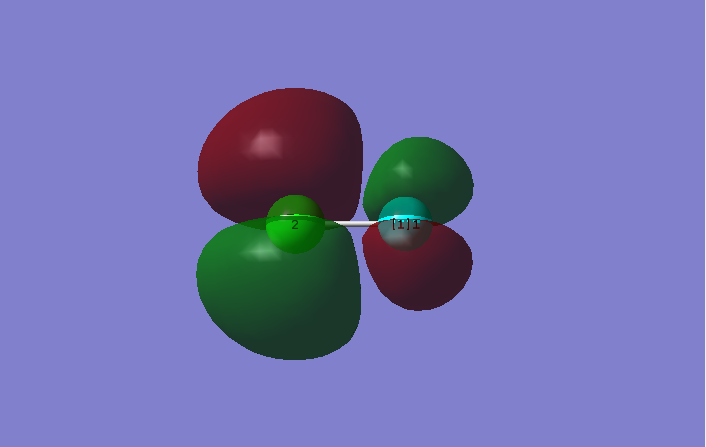
This is an occupied antibonding combination between 3p orbital in Cl and 2p orbital in F. The molecular orbital is the HOMO. Decreasing electron density between nuclei weaken the bonds.

This is an occupied bonding combination between 3p orbital in Cl and 2p orbital in F.