Rep:MOD:LYF1996
Introduction
The transition state in potential energy surface would be a stationary point which is the highest point on the reaction energy curve. It means the just point of the reactant is forming their bonds or breaking their bonds to form the product. However, a stationary point is not always the transition state. It can still be the minimum energy point which means physically stable point. That is the minimum point on the potential energy surface. During a frequency calculation, the negative frequency is the transition state which is called as an imaginary frequency. But, if only real frequencies exist, that is a minimum structure for the molecule.
Nf710 (talk) 12:27, 4 November 2016 (UTC) You should talk about house TS and minimums are stationary points with zero gradients wrt to the energy and and all degrees of freedom. which are distinguished by their second derivatives.
First part write up
In this part a Diels-Alder reaction (Butadiene with Ethylene) is analysed about its MO diagram, bond length and bond formation at the reactant, product and transition state.
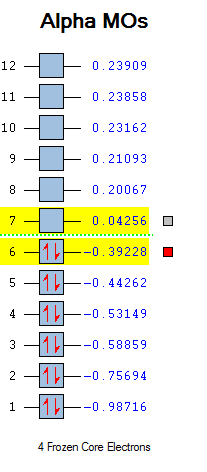
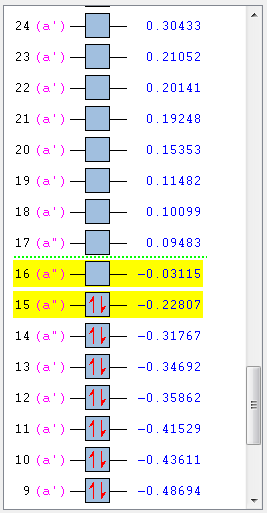
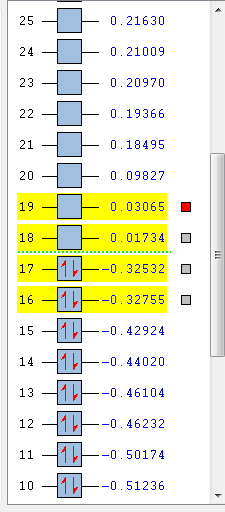
First comes to the MO. The MO image of the reactant and transition state are listed below. The shape of transition state can be found from the four reactant MO graph if the whole MO is read from each partial MO. Transition State 16 is formed from the HOMO of butadiene and LUMO of ethylene. Transition State 17 is formed from the HOMO of ethylene and LUMO of butadiene. Transition State 18 is formed from the HOMO of ethylene and LUMO of butadiene. Transition State 19 is formed from the HOMO of butadiene and LUMO of ethylene. So, only same symmetry can be used to form a MO. If the two reactant are at the same symmetry, the overlap integral would be non-zero or it would be cancelled out to give zero integral. This means a gerade-ungerade interaction gives zero overlap integral, a gerade-gerade interaction gives a non-zero integral and an ungerade-ungerade interaction gives a non-zero integral.
Nf710 (talk) 12:33, 4 November 2016 (UTC) All of this could be explained with a traditional MO diagram. But i see what you are trying to do.
 |
 |
 |
|---|
From the MO chart, it is easily compared and can be told that this is a neutral Diels-Alder reaction.
Then comes to the bond length analysis. A labelled graph and a table for the bond length for the C-C bond are shown below. The standard Sp2 and Sp3 C-C bond length are 1.47 Å and 1.54Å respectively. (1) And, the Van der Waals radius of carbon atom is 1.7 Å, which means 3.4 Å for two atoms

| It is shown that the bond length except between carbon 2 and
3 are increasing. If we compare the length values with the standard sp2 and sp3 c-c length, it is easily found that merely carbon-carbon bond between 2 and 3 are turning into a sp2 hybridised one while the other bond are changing from the sp2 like into sp3 like. As for the Van der Waals radius comparison between two carbon atoms and the carbon-carbon distance between atom 4 to 5 and 6 to 1, we can found that the two bond length are both smaller than the Van der Waals radius between two carbon atoms. This shows there is bond forming between the two carbons or they should equal or larger than the Van der Waals radius. Finally, the vibration that corresponds to the reaction path at the transition state which is at the only negative frequency. The two end carbon atoms of butadiene and the two atoms of the ethylene are vibrating in an opposite direction. 
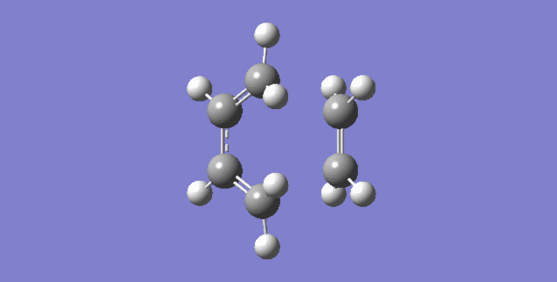
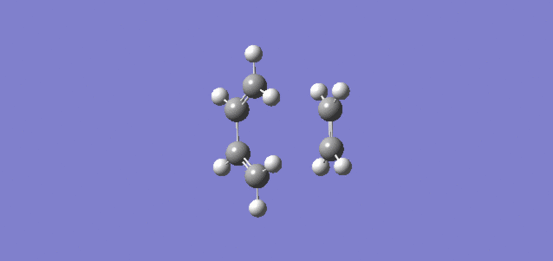 Nf710 (talk) 12:58, 4 November 2016 (UTC) This was answered fairly well. YOu could have put in an actual MO diagram. The negative frequency is the reaction cordinate that you go along to get from reactant to products and therefore. this is the bond forming process. and it can be seen that the bond formation is synchronous.
Second part write upFirst, comes to the MO part. Both the transition state of ENDO and EXO process are listed below.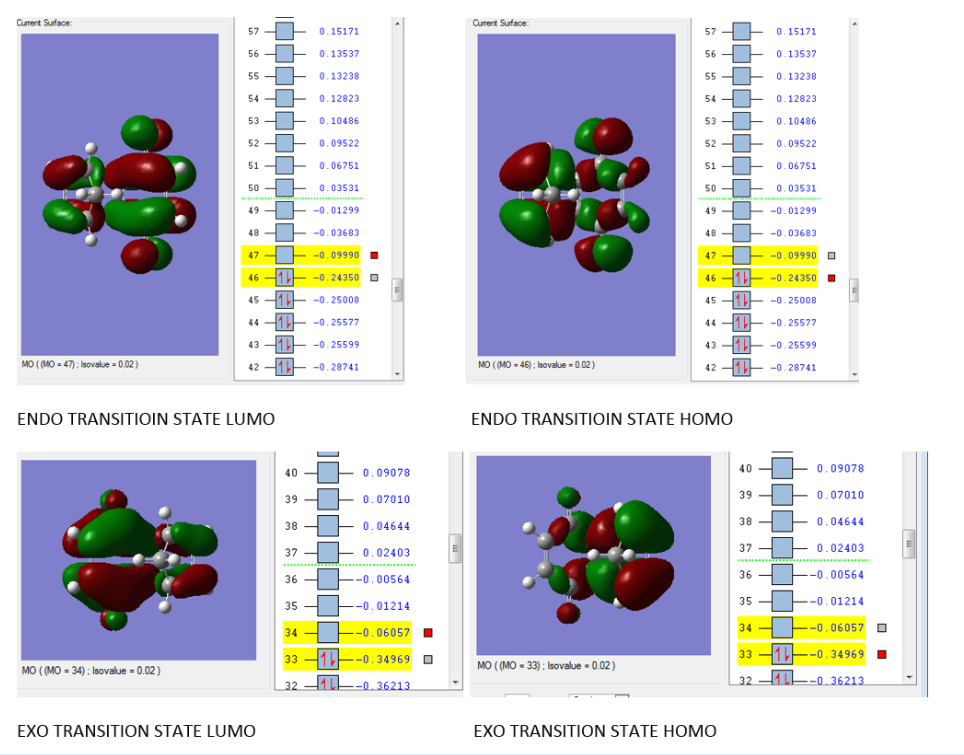  diagram of the two reactants, the cyclopentadiene as diene role has higher energy of both HOMO and LUMO then the Benzoquinone as an ene role. So, it tells that the reaction is a normal electron demand reaction. interaction only takes place in ENDO Transition state but not in EXO transition state. From the Endo transition state MO diagram, the two part side orbital turn into a bit twist like. So, it could be concluded as a secondary interaction and will act as a stabilization that lower barrier energy (2). Nf710 (talk) 13:20, 4 November 2016 (UTC) Your energies look abit off, did you use the correct basis set, I cant check your file to have a look. Also you haven't stated the kenetic or thermo product. And have only said that the endo has a lower energy barrier. |
Third part write up
Both the Diels-Alder and Cheletropic IRC route are listed below.If they are considered from the product side to the reactant side as the analysis done, the delocalised electron density from the six membered ring is withdraw from the ring system during the reaction to form the two outer double bond. This means inside the ring, six delocalised pi bonds are changing into three discrete sp2 bonding. If they are considered as the reactants to products, the electrons from oxygen and sulfur atom is forming bond with the two outer carbon atoms and push electron( from double bond ) into the ring and formed a delocalised system.
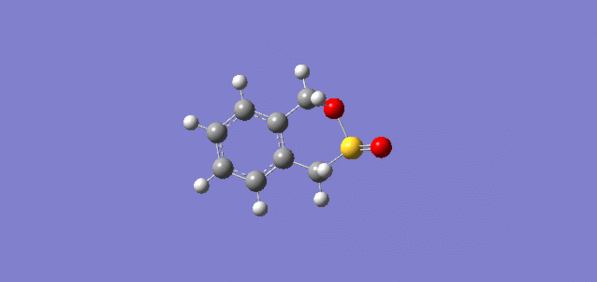
Diels-Alder route
(This is the exo-Diels Alder reaction. The endo will have the oxygen overlapping the diene Tam10 (talk) 17:31, 27 October 2016 (BST))
Cheletropic route
The free energy data are list as table below


So, from the activation energy comparison and reaction energy comparison, it is known that Diels-Alder route is kinetically favored and cheletropic route is thermal dynamically favored.
As for the Cheletropic route, there is only exothermic reaction. The reaction profile is listed below.
(What does this mean? Endo and exo are not the same as endothermic and exothermic Tam10 (talk) 17:31, 27 October 2016 (BST))

For the Diels-Alder route, both exo and endo type of route exist. They are listed below.

Exo of Diels-Alder route

Endo of Diels-Alder route
(These would be far more useful if they were shown on the same diagram! Tam10 (talk) 17:31, 27 October 2016 (BST))
Conclusion
From the analysis of three Diels-Alder reactions, method of frequency calculation, optimization and IRC were used to carry out the data of potential surface, MO diagram, free energy and reaction pathway. It is found that the transition state is the highest energy point on the reaction energy pathway with a negative frequency in the frequency calculation. And, the potential energy surface, PES is a surface that combine many parameter that can describe the energy profile. During the endo reaction, the extra secondary orbital interaction (from MO) and steric are often found to stabilise the product which makes the product thermodynamically favoured.
Reference
1) Fox, Marye Anne; Whitesell, James K. (1995). Organische Chemie: Grundlagen, Mechanismen, Bioorganische Anwendungen. Springer. ISBN 978-3-86025-249-9.
(2) Governing organic reactions through secondary orbital interactions. Semiempirical and density functional theory study of catalyzed cycloaddition reactions between pyrrole and ether dienophiles
Branko S. Jursic Department of Chemistry, University of New Orleans, New Orleans, Louisiana 70148, USA Received (in Gainesville, FL) 18th May 1998, Accepted 7th December 1998