Rep:MOD:JSComplab
NH3 Molecule
Molecule Summary
Molecule Name: Ammonia, NH3
Calculation Method: B3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -56.55776873 au
Point Group: C3V
N-H Bond Length: 1.01798 Å
H-N-H Bond Angle: 105.741 Degrees
"Item" table from the *.log file
Item Value Threshold Converged? Maximum Force 0.000346 0.000450 YES RMS Force 0.000227 0.000300 YES Maximum Displacement 0.001056 0.001800 YES RMS Displacement 0.000512 0.001200 YES
The optimisation file is linked to here
Dynamic Image of NH3
Ammonia |
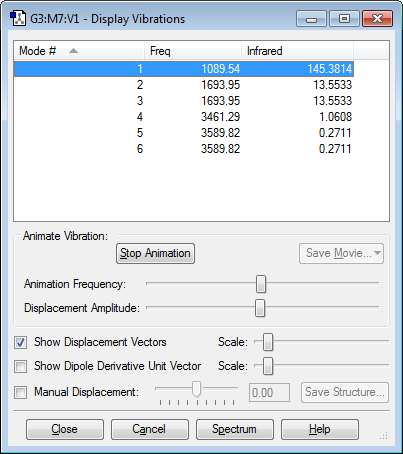
Vibrational Modes of NH3 Molecule
Questions
1) How many modes do you expect from the 3N-6 rule? N = 4 Therefore 6 modes expected 2) Which modes are degenerate (ie have the same energy)? 2&3 and 5&6 3) Which modes are "bending" vibrations and which are "bond stretch" vibrations? 1,2 & 3 “bending” vibrations, 4,5 & 6 are “bond stretch” vibrations 4) Which mode is highly symmetric? 4 5) One mode is known as the "umbrella" mode, which one is this? 1 6) How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2
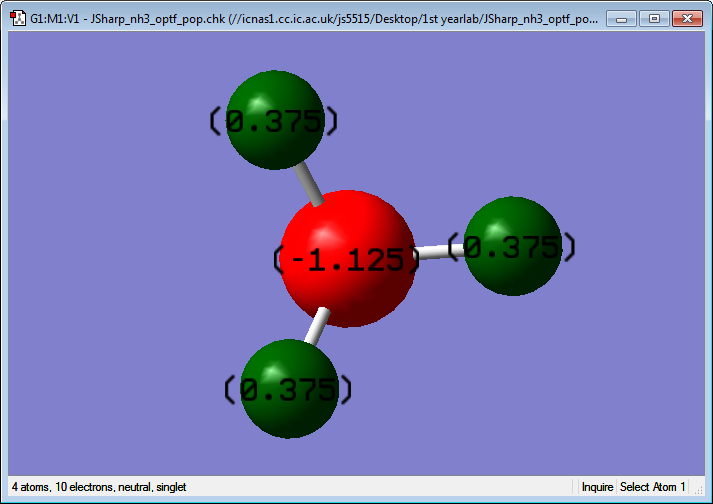
Atomic Charge
Charge on N: -1.125
Charge on H: 0.375
This is to be expected as Nitrogen is much more electronegative than Hydrogen.
N2 Molecule
Molecule Summary
Molecule Name: Nitrogen, N2
Calculation Method: B3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -109.524 au
Point Group: D∞h
N-N Bond Length: 1.0550 Å
"Item" table from the *.log file
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked to here
Dynamic Image of N2
Nitrogen |
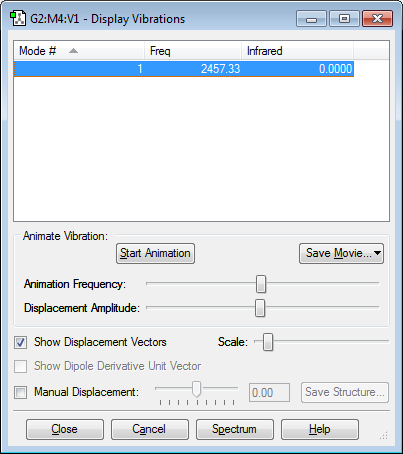
Vibrational Modes of N2 Molecule
Questions
1) How many modes do you expect from the 3N-6 rule? N = 2 Therefore 1 mode expected 2) Which modes are degenerate (ie have the same energy)? N/A 3) Which modes are "bending" vibrations and which are "bond stretch" vibrations? This mode is a “bond stretch” vibration 4) Which mode is highly symmetric? 1 5) One mode is known as the "umbrella" mode, which one is this? N/A 6) How many bands would you expect to see in an experimental spectrum of gaseous nitorgen? 0, as the vibration is symmetrical there is no change in dipole moment
Atomic Charge
As can be seen, the charge on the Nitrogen atoms is 0. This is expected as the two atoms are the same and therefore there is no difference between the electronegativities, the electron distribution is equal.
H2 Molecule
Molecule Summary
Molecule Name: Hydrogen, H2
Calculation Method: B3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -1.17853936 au
Point Group: D∞h
H-H Bond Length: 0.74279 Å
"Item" table from the *.log file
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked to here
Dynamic Image of H2
Hydrogen |
Vibrational Modes of H2 Molecule
Questions
1) How many modes do you expect from the 3N-6 rule? N=2, therefore 1 mode expected 2) Which modes are degenerate (ie have the same energy)? N/A 3) Which modes are "bending" vibrations and which are "bond stretch" vibrations? This mode is "bond stretch" 4) Which mode is highly symmetric? 1 5) One mode is known as the "umbrella" mode, which one is this? N/A 6) How many bands would you expect to see in an experimental spectrum of gaseous Hydrogen? Ad the vibration is symmetrical, there is no dipole moment and therefore 0 bands would be seen.
Atomic Charge
As can be seen, the charge on the Hydrogen atoms is 0. This is expected as the two atoms are the same and therefore there is no difference between the electronegativities, the electron distribution is equal.
Reactivity of Ammonia
N2 + 3H2--> 2NH3
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.1155375 a.u.
E(N2)= -109.52412867 a.u.
E(H2H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074 a.u.
ΔE= -0.05579074 x 2625.5 = -146.4785879 kJ/mol
= -146.48 kJ/mol
A value of -146.48 kJ/mol shows that energy is released when ammonia is formed. This shows that is more stable than the starting reactant molecules.
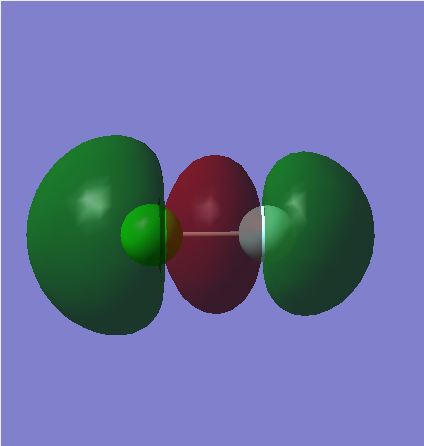
ClF Molecule
Molecule Summary
Molecule Name: ClF
Calculation Method: B3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -559.94269578 au
Point Group: D∞h
Cl-F Bond Length: 1.66434 Å
"Item" table from the *.log file
Item Value Threshold Converged? Maximum Force 0.000246 0.000450 YES RMS Force 0.000246 0.000300 YES Maximum Displacement 0.000443 0.001800 YES RMS Displacement 0.000613 0.001200 YES
The optimisation file is linked to here
Dynamic Image of ClF
ClF |
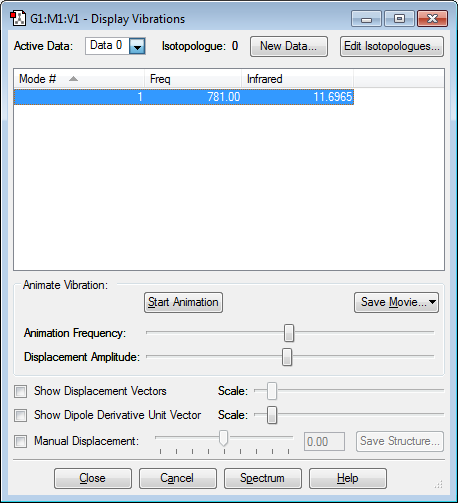
Vibrational Modes of ClF Molecule
Questions
1) How many modes do you expect from the 3N-6 rule? N = 2 Therefore 1 mode expected 2) Which modes are degenerate (ie have the same energy)? N/A 3) Which modes are "bending" vibrations and which are "bond stretch" vibrations? This mode is 'bond stretch' 4) Which mode is highly symmetric? 1 5) One mode is known as the "umbrella" mode, which one is this? N/A 6) How many bands would you expect to see in an experimental spectrum of gaseous ClF? 0
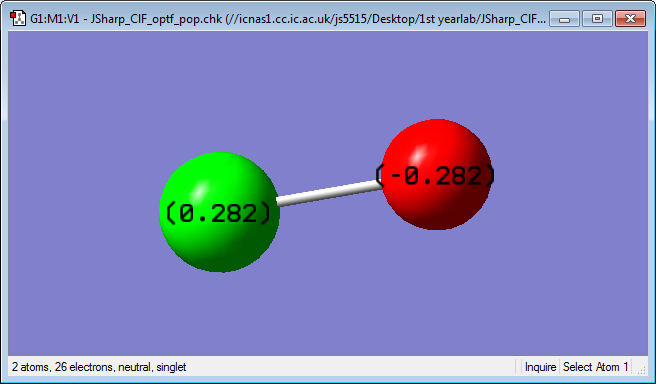
Atomic Charge
Charge on Cl: 0.282
Charge on F: -0.282
The charges on the Cl and F are equal but opposite, this is due to the oxidation states also being equal and opposite, Cl at +1 and F at -1. The negative charge on the F atom is due to it's high electronegativity pulling electron density towards itself. Cl has a higher quantum number than F and therefore a higher level of shielding is experienced by the valence electrons and they are not held as closely the nucleus.
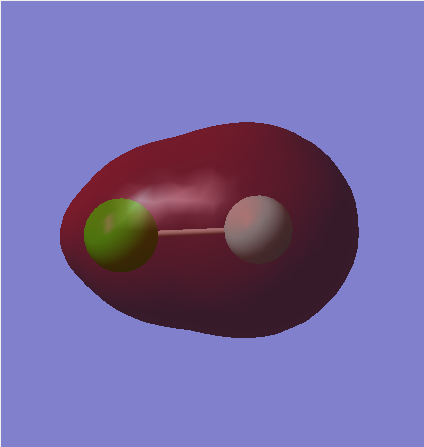
Molecular Orbitals
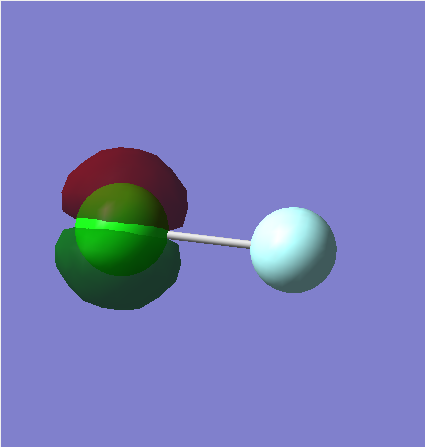
MO1
This is a non-bonding 2p orbital. There are three filled non-bonding 2p orbitals on the Cl atom, each on a differing axes. The image only has electron density around the Cl atom as the orbital is of too low an energy to contribute in the bonding MO's. The differing colours show the in-phase and out of phase parts of the orbitals.
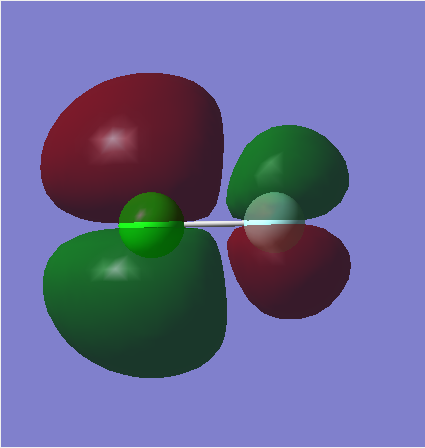
MO2
Here, a filled pi bonding orbital can be seen. This is formed from the overlap of the 2p F orbital and the 3p Cl orbital. Both the p orbitals are in phase and therefore overlap to form a bonding orbital. However, no pi bond is formed here due to the fact that the corresponding anti-bonding orbital is also filled.
MO3
This is the filled pi-anti bonding orbital. There is no constructive overlap as the orbital lobes are not in the same phase. Due to the fact this MO is filled, there is no pi bond and the ClF molecule is of bond order 1 with a single sigma bond.
MO4
This is the MO orbital corresponding to the single sigma bond in the ClF molecule. This is the overlap occurring between the 2p F atomic orbital and the 3p Cl atomic orbital. The in-phase part of the p orbitals overlap head on to form this sigma bonding orbital. The corresponding anti-bonding orbital is the LUMO. As this anti-bonding orbital is not filled the single bond is formed here.
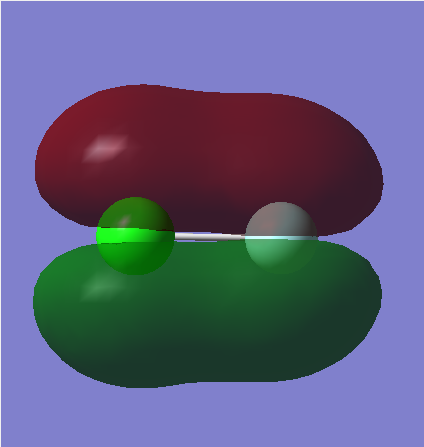
MO5
Here, the 2s F atomic orbital and 3s Cl atomic orbital overlap to form this is the bonding MO orbital. The orbitals are in the same phase and therefore this is a sigma bonding orbital, however a bond is not formed here due to the fact the corresponding sigma antibonding orbital is also filled.
HF Molecule
Molecule Summary
Molecule Name: Hydrogen Fluoride, HF
Calculation Method: B3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -100.42746153 au
Point Group: D∞h
H-F Bond Length: 0.92540 Å
"Item" table from the *.log file
Item Value Threshold Converged? Maximum Force 0.000019 0.000450 YES RMS Force 0.000019 0.000300 YES Maximum Displacement 0.000016 0.001800 YES RMS Displacement 0.000022 0.001200 YES
The optimisation file is linked to here
Dynamic Image of HF
HF, Hydrogen Fluoride |
Vibrational Modes of HF Molecule
Questions
1) How many modes do you expect from the 3N-6 rule? N = 2 Therefore 1 mode expected 2) Which modes are degenerate (ie have the same energy)? N/A 3) Which modes are "bending" vibrations and which are "bond stretch" vibrations? This mode is 'bond stretch' 4) Which mode is highly symmetric? 1 5) One mode is known as the "umbrella" mode, which one is this? N/A 6) How many bands would you expect to see in an experimental spectrum of gaseous HF? 0
Atomic Charge
Charge on H: 0.543
Charge on F: -0.543
The charges on the H and F are equal but opposite, this is due to the oxidation states also being equal and opposite, H at +1 and F at -1. The negative charge on the F atom is due to it's high electronegativity pulling electron density towards itself.