Rep:MOD:JG1C
Conformational Analysis of Dicyclopentadiene hydrogenation and Taxol Intermediate
Dicyclopentadiene Hydrogenation
Optimisation of Endo and Exo Dicyclopentadiene Conformers
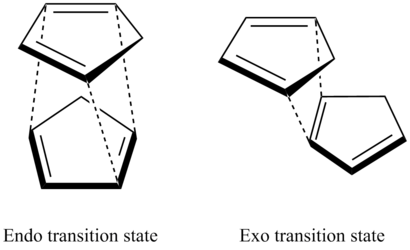
All MMFF94 optimisation calculations were performed to a minimum RMS gradient of 0.001. Cyclopentadiene readily dimerises via π 4s + π 2s or π 2s + π 4s Diels Alder cycloadditions. This results in the formations of two conformers, exo and endo. Both products can form via two routes; the reaction between the diene on cyclopentadiene 1 and alkene on cyclopentadiene 2 but also vice versa. As this is a dimerisation, the two endo products are indistinguishable unless a deuterium labelled cyclopentadiene used. [1]
Using the molecular modelling MMFF94 calculations, energies of 55.3731 kcal mol-1 and 58.1902 kcal mol-1 were found for the exo and endo products respectively. The lower energy of the exo product indicates its relative thermodynamic stability with respect to the endo product. However, Diels Alder dimerisations of cyclopentadiene result almost exclusively in the endo product,[2] suggesting that the reaction is kinetically controlled.
This is validated by previous ab initio calculations that indicate a lower activation energy for the endo transition state by 2.56 kcal mol-1. [3] The stability of the endo transition state is a result of its bispericyclic nature.[4] Both transition states are shown in figure 1. Secondary orbital interactions in the exo TS provide this stabilisation and reduce its energy to make it the favourable kinetic product.
Exo dicyclopentadiene product |
Endo dicyclopentadiene product |
Optimisation of Dicyclopentadiene Hydrogenation Products

The hydrogenation of dicyclopentadiene (DCPD) is an important reaction for the industrial synthesis of artificial jet propellant 10 - a fuel that is used in multiple missile systems.[5] As DCPD was recognised as a promising feedstock for organic synthesis, copious research emerged to find an efficient catalytic system; uncatalysed hydrogenation requires high temperatures and risks cracking DCPD into monomeric cyclopentadiene.[6]
Dicyclopentadiene has two C=C unsaturations: one in the norbornene ring and the other in the cyclopentene ring. As the exo-conformer of DCP is the most common, its hydrogenation products were chosen to be modelled. Hydrogenation of the cyclopentene olefin lead to product 1 with an energy 50.7225 kcal mol-1. Hydrogenation of the norbornene ring olefin lead to product 2 with 41.2575 kcal mol-1.
Re-optimisation of these hydrogenation products 1 and 2 in Avagadro resulted in energy values of 50.72251 kcal mol-1 and 41.25821 kcal mol-1 respectively. Their deviations from ideality are tabulated below.
| Hydrogenation Product | Total ... energy (kcal mol-1) | |||||
|---|---|---|---|---|---|---|
| Bond stretching | Angle bending | Torsional | Van der Waals | Electrostatic | Molecular | |
| 1 | - 1.92653 | 0.05715 | 0.01340 | 13.27666 | 5.12069 | 50.72251 |
| 2 | - 1.65645 | - 0.37110 | 0.00029 | 10.62767 | 5.14711 | 41.25821 |
The molecular modelling eluded that the hydrogenation product 2 was the more stable. This was a result of the greater ring strain caused by sp2 centres in the norbornene ring compared to that of the cyclopentene ring.[7] Relief of this greater strain by hydrogenation lead to the lower energy product. This was demonstrated by the data in table 1; there were significantly lower deviations for total angle bending, torsional and van der Waals energies for product 2.
Intermediate of Taxol Synthesis
Taxol was first extracted from the bark of Pacific yew trees before its' anti-tumour properties were discovered.[8] Taxol has had a controversial history. There was fierce competition in the 1990s to find a total synthesis to produce the complex stereochemical compound and controversy when Bristo-Myers Squibb trademarked the name "Taxol".[9] The active ingredient, Paclitaxel, has been used to treat breast and ovarian cancer in both the UK and US.
The oxy-cope rearrangement of the taxol intermediate can lead to the formation of a ketone. This ring can rotate, about the less substituted C-C bond adjacent to the carbonyl, to give boat and chair like conformers with the carbonyl up or down respectively.[10] This examples atropisomerism; axial stereochemistry where the rotational strain about a single bond is large enough to allow individual conformers to be isolated.[11] Historically biaryl compounds have best demonstrated atropisomerism.[12]
| Compound | Carbonyl Position | Energy / kcal mol-1 |
|---|---|---|
| 9 | Up | 76.27201 |
| 10 | Down | 66.34763 |
When modelling these atropisomers, manual edits were required to ensure that the alkene orientated to give the E-cyclononenone ring. Additionally the ethyl bridge had to be manually positioned on the bottom face of the E-cyclononenone ring. Without these manual edits the structure preferentially isomerised to the Z-cyclononenone ring.
Resultant MMFF94s force field calculations eluded that compound 10 was significantly more stable than atropisomer 9. This large energy difference arose from the strained conformation of the ring. Compound 10 adopted a chair like conformation where as compound 9 adopted a twist boat one.[10] The understanding and analysis of the conformation of E-cyclononene rings has been limited, especially when compared to that of Z-cyclononene ring.[13] Interconversion between E and Z isomers occurs readily at room temperature but is prevented in this Taxol intermediate by the ethyl bridge. Computational research has shown that the chair conformer of E-cyclononene is the most stable conformer where as the twist boat is a higher energy minimum.[14] These conformational properties propagated through to the Taxol structure, explaining the energy difference between 9 and 10.
Compound 9 - carbonyl up atropisomer |
Compound 10 - carbonyl down atropisomer |
This intermediate is heavily related to that for which atropisomerism was discussed. The two difference in the structure are a methyl group on the ring juncture and the dithiolane group on the fused cyclohexane. These act to provide greater strain and further lock both rings into either the twist boat or chair conformer. [10] Calculation of the overall energy of compound 18 was found to be 102.21310 kcal mol-1. This demonstrates the greater strain in the compound as it is energy is almost double that of analogous structure, compound 10.
The structures of large natural molecules can be difficult to determine from pure experimental data. Although crystal structure often provide a definitive orientation of a molecule, not all molecules can be crystalised to a high enough quality for analysis. Here ab initio calculations form a bridge between complex organic chemistry and computational. In the past, proposed structures have been shown to be incorrect by analysing quantum mechanically predicted NMR spectra.[15]
Comparison of the computed data to literature indicated that the correct conformer had be drawn.[16] However, the data was occasionally out by a 2-3 ppm but this was deemed insignificant. Atropisomers, like compounds 17 and 18, have slightly differing NMR spectra due to the different arrangement of atoms in 3 dimensions. This leads to differing chemical shifts and coupling constants.
Compound 18 - carbonyl down Taxol related intermediate |
 |
 |
 |

 |
 |
 |
| Energy / Hartrees particle-1 | |
|---|---|
| Zero-point correction | 0.467688 |
| Thermal correction to Energy | 0.489219 |
| Thermal correction to Enthalpy | 0.490163 |
| Thermal correction to Gibbs Free Energy | 0.420441 |
| Sum of electronic and zero-point Energies | - 1651.415538 |
| Sum of electronic and thermal Energies | - 1651.394008 |
| Sum of electronic and thermal Enthalpies | - 1651.393063 |
| Sum of electronic and thermal Free Energies | - 1651.462785 |
NMR Calculation: DOI:10042/161063
Computational Analysis of the Selectivity of Jacobsen and Shi Catalysted Epoxidations
Structural Analysis of Catalysts and Epoxides
Shi and Jacobsen Crystal Structures
Crystal structures of both catalysts display interesting features. Within the organic Shi catalyst, the unsymmetrical anomeric effects leads to the contraction of C-O bonds. This is demonstrated in the crystal structure (figure 4). [17] The anomeric effect consists of the stereoelectric donation of an oxygen lone pair into a sigma star C-O antibonding orbital (nOsp3 -> σ*C-O). This effect is maximised when the orbitals orientate anti-periplanar to each other. In sugars, DFT ab initio calculations have demonstrated C-O bond contraction of up to 0.04 Å when there is efficient overlap.[18] The Shi catalyst structure correlates strongly with this as it is a derivative of D-fructose.
The asymmetrical anomeric effects manifest in the Shi catalyst because C-O bonds have very little rotational freedom - fixing the orientation of the interacting orbitals. The five membered hetero aromatic ring on C14 have the greatest anomeric effects in the structure as they can interact with the C14-O12 bond of the pyranose ring.
The strongest anomeric effect are:
- (nO7sp3 -> σ*C19-O8)
- (nO8sp3 -> σ*C14-O12)
These resulted in contracted C-O bond lengths of 1.380 Å and 1.390 Å as the bonds had a greater double bond character.

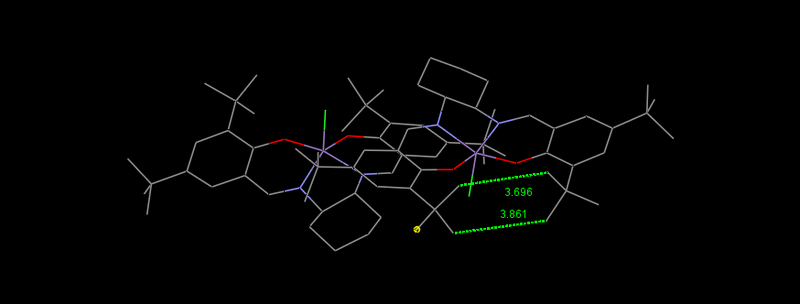
Within the Jacbosen crystal structure,[19] the proximity of the t-butyl groups are of particular interest. The sum of van der Waals radii would indicate that these groups would undergo significant steric clashing. However these ortho t-butyl groups, along with the para t-butyl groups, provide the stereoselectivity of the catalyst as they force olefins to approach over the diimmine bridge.[20]
NMR analysis of styrene and trans-β-methyl styrene epoxides
R - styrene oxide |
R,R - trans beta-methylstyrene oxide |
S - styrene oxide |
S,S - trans beta-methylstyrene oxide |
Using a reference of TMS B3LYP/6-311+G(2d,p) GIAO for all spectra, the data was analysed and showed the correct chemical shifts and integrations for the epoxides.
R - styrene oxide
 |
 |
 |
R,R - methyl styrene oxide
 |
 |
 |
 |
S - styrene oxide
 |
 |
 |
S,S - methyl styrene oxide
 |
 |
 |
 |
Assigning Absolute Stereochemistry
Optical Rotation of Epoxides
Measuring the optical rotation of a product is important for both characterisation and purity assessment. Absolute optical rotary values can be used to determine the composition of a mixture of enantiomers if the absolute value for a pure enatiomer is know.
Unfortunately, insufficient time was available to collect optical rotary values for the epoxides synthesised in the laboratory. Instead the techniques used to assign absolute stereochemistry for compounds will be scrutinised by completing computations for assigned enantiomers of styrene oxide and trans-β-methylstyrene oxide.
The optical rotary powers of these two epoxides were computed quantum mechanically using density functional theory. In order to ascertain comparable data, computations were completed with the following parameters:
- Cambridge B3LYP basis set
- Wavelength 589.00 nm
- Chloroform solvent
- Specific rotation was recorded
To test that the computed optical values were correct, the calculation was repeated on the other enantiomer with the expectation of finding a value of the same magnitude but of the opposite sign. A summary of these calculations is visible in table 4.
| Compound | Absolute stereochemistry | Computed optical rotary power / deg. | Literature optical rotary power / deg. |
|---|---|---|---|
| Styrene oxide | R | - 30.44 | - 33.3 [21] |
| S | 30.08 | 32.1 [22] | |
| Trans-β-methylstyrene oxide | R,R | 46.77 | 46 [23] |
| S,S | - 46.92 | - 43.6 [23] |
The recorded values were deemed to be correct as enantiomers had similar rotation in the opposite direction. However, for the R - styrene oxide the light was computed to rotate left rather than the expected right and vice versa for the S - enantiomer. Furthermore, there was more evidence to question the viability of this technique. Although literature values were found that correlated strongly to calculated values, there were multiple reported values of different magnitudes and signs for the same enantiomer. This is despite the fact that specific rotation is an intensive property.
Issues arise with absolute rotary values as they are only recorded at a single wavelength. If the wavelength used is far away from the maximum absorption of the molecule, the values are smaller and contain more error. The technique of optical rotatory dispersion solves this problem by scanning at different wavelength. Simply using a DFT calculation and polarimeter, large organic molecules can be assigned absolute configurations easily. [24]
DOIs for these computations can be found in the appendix.
Analysis of Transitions States for the Catalysed Epoxidations
DFT calculations of the transition states allowed analysis of the selectivity of both catalyst. By comparing the energy and therefore the stability of the transition states for each conformer predictions could be made about the ee values that would be achieved in the epoxidations.
Tabulated below are all the energies computed. Unfortunately, a different basis set was used to calculate the total energies of the starting reagents and products compared to the transition states so the energies aren't comparable. However, enatiomeric excess can still be predicted using kinetic resolutions.
| Compound | Energy of DFT optimised structure / Hartrees | DOI |
|---|---|---|
| Styrene | - 309.66090577 | DOI:10042/161099 |
| Trans β-methylstyrene | - 348.98331321 | DOI:10042/161100 |
| R - styrene oxide | - 384.85822315 | DOI:10042/161664 |
| R,R - trans β-methylstyrene oxide | - 424.18347471 | DOI:10042/161101 |
| S - styrene oxide | - 384.85822316 | DOI:10042/161674 |
| S,S - trans β-methylstyrene oxide | - 424.18347470 | DOI:10042/161680 |
| Transition state energy / Hartrees | |||||
|---|---|---|---|---|---|
| Transition state 1 | Transition state 2 | Transition state 3 | Transition state 4 | ||
| Shi catalysed epoxidations | R - styrene oxide | - 1303.730703 | - 1303.730238 | - 1303.736813 | - 1303.738044 |
| R,R - trans β-methylstyrene oxide | - 1342.942109 | - 1342.935636 | - 1342.948578 | - 1342.951773 | |
| S - styrene oxide | - 1303.654184 | - 1303.646003 | - 1303.650626 | - 1303.658890 | |
| S,S - trans β-methylstyrene oxide | - 1342.936287 | - 1342.934660 | - 1342.941918 | - 1342.942317 | |
| Jacobsen catalysed epoxidation | R - styrene oxide | - 3343.848196 | - 3343.849686 | - | - |
| R,R - trans β-methylstyrene oxide | - 3383.137053 | - 3383.141188 | - | - | |
| S - styrene oxide | - 3343.855840 | - 3343.850491 | - | - | |
| S,S - trans β-methylstyrene oxide | - 3383.145943 | - 3383.142153 | - | - | |
These values are made more representative in semi quantitative figures 10 - 13. From the reaction coordinate, it is evident that Shi catalyst provides R selectivity, which seems to be almost exclusive for styrene but not for trans β-methylstyrene. Conversely, Jacobsens shows selectivity for the formation of S conformers. These relationships do not correlate to literature where the Shi catalysed shows higher selectivity for trans olefins compared to terminal and cis olefins.[25]
To predict product ee values the equation [26] where Kr is the rate constant calculated by using ΔΔG‡, the difference between the ΔG‡ of forming each enantiomer.
| Shi catalysed epoxidation | Jacobsen catalysed epoxidation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔΔG‡ taken between | ΔΔG‡ / Joules | Kr | Product ee | ΔΔG‡ taken between | ΔΔG‡ / Joules | Kr | Product ee | ||
| Styrene | Highest predicted ee | RTS#4 & STS#2 | 4.012755840762e-19 | 1 | 100% | ||||
| Lowest predicted ee | RTS#2 & STS#4 | 3.1105931457359995e-19 | 1 | 100% | |||||
| Trans β-methylstyrene | Highest predicted ee | R,RTS#4 & S,STS#2 | |||||||
| Lowest predicted ee | |||||||||
Untested Epoxide
The epoxide chosen for further research would be anti-benzene dioxide. This molecule is of particular interest as it is small (MW = 110.112), is derived from benzene, has dual epoxy functionality, which generates 4 stereogenic centres. Additionally, there has only been a few reported optical rotation for the compound of 320.6 deg. which may well be incorrect. [27]
Appendix
DOI for HPC Calculations
| Epoxide | HPC calculation | |||
|---|---|---|---|---|
| NMR | Optical Rotary Power | Electronic Circular Dichroism | Vibrational Circular Dichroism | |
| R - styrene oxide | DOI:10042/161064 | DOI:10042/161066 | DOI:10042/161071 | DOI:10042/161073 |
| R,R - methyl styrene oxide | DOI:10042/161065 | DOI:10042/161067 | DOI:10042/161072 | DOI:10042/161074 |
| S - styrene oxide | DOI:10042/161082 | DOI:10042/161093 | DOI:10042/161095 | DOI:10042/161097 |
| S,S - methyl styrene oxide | DOI:10042/161083 | DOI:10042/161094 | DOI:10042/161096 | DOI:10042/161098 |
References
- ↑ J. E. Baldwin, "Ab initio study of Diels-Alder reactions of cyclopentadiene with ethylene, isoprene, cyclopentadiene, acrylonitrile, and methyl vinyl ketone", J. Org. Chem., 1966, 31, 2441–2444. DOI:10.1021/jo01346a003
- ↑ P. Caramella, P. Quadrelli and L. Toma, "An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the Endo Dimerization of Cyclopentadiene", J. Am. Chem. Soc., 2002, 124, 1130–1131. DOI:10.1021/ja016622h
- ↑ W. L. Jorgensen, D. Lim and J. F. Blake, "Ab initio study of Diels-Alder reactions of cyclopentadiene with ethylene, isoprene, cyclopentadiene, acrylonitrile, and methyl vinyl ketone", J. Am. Chem. Soc., 1993, 115, 2936–2942. DOI:10.1021/ja00060a048
- ↑ J. Limanto, K. S. Khuong, K. N. Houk and M. L. Snapper, "Intramolecular Cycloadditions of Cyclobutadiene with Dienes: Experimental and Computational Studies of the Competing (2 + 2) and (4 + 2) Modes of Reaction", J. Am. Chem. Soc., 2003, 125, 16310–16321. DOI:10.1021/ja0380547
- ↑ J.-J. Zou, X. Zhang, J. Kong and L. Wang, "Hydrogenation of Dicyclopentadiene over amorphous nickel alloy catalyst SRNA-4", Fuel, 2008, 87, 3655–3659. DOI:10.1016/j.fuel.2008.07.006
- ↑ T. N. Antonova, I. a. Abramov, V. S. Feldblyum, I. G. Abramov and a. S. Danilova, "Catalytic hydrogenation of dicyclopentadiene to dicyclopentene in the liquid phase", Pet. Chem., 2009, 49, 366–368. ]DOI:10.1134/S0965544109050041
- ↑ P. R. Khoury, J. D. Goddard and W. Tam, "Ring strain energies: substituted rings, norbornanes, norbornenes and norbornadienes", Tetrahedron, 2004, 60, 8103–8112. DOI:10.1016/j.tet.2004.06.100
- ↑ M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon and A. T. McPhail, "Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia" , J. Am. Chem. Soc., 1971, 93, 2325–2327. DOI:10.1021/ja00738a045
- ↑ "Creating complexity - the beauty and logic of synthesis", Chem. Commun., 2003, 661–664. DOI:10.1039/B212248K
- ↑ 10.0 10.1 10.2 S. W. Elmore and L. A. Paquette, "The first thermally-induced retro-oxy-cope rearrangement", Tetrahedron Lett., 1991, 32, 319–322. DOI:10/dqw8k7
- ↑ G. Bringmann, A. J. Price Mortimer, P. A. Keller, M. J. Gresser, J. Garner and M. Breuning, "Atroposelective Synthesis of Axially Chiral Biaryl Compounds", Angew. Chemie Int. Ed., 2005, 44, 5384–5427. DOI:10.1002/anie.200462661
- ↑ G. H. Christie and J. Kenner, "LXXI.-The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of [gamma]-6 : 6[prime]-dinitro- and 4 : 6 : 4[prime or minute] : 6[prime]-tetranitro-diphenic acids into optically active components", J. Chem. Soc. Trans., 1922, 121, 614–620. DOI:10.1039/CT9222100614
- ↑ D. M. Pawar, S. D. Miggins, S. V Smith and E. A. Noe, "Conformational Study of cis-Cyclononene by Dynamic NMR Spectroscopy and Computational Methods", J. Org. Chem., 1999, 64, 2418–2421. DOI:10.1021/jo982226e
- ↑ I. Yavari, H. Kabiri-Fard and S. Moradi, "AM1 study of conformational properties of (E)-cyclononene", J. Chem. Res., 2000, 384–385. DOI:10.3184/030823400103167769
- ↑ M. W. Lodewyk, C. Soldi, P. B. Jones, M. M. Olmstead, J. Rita, J. T. Shaw and D. J. Tantillo, "The Correct Structure of Aquatolide—Experimental Validation of a Theoretically-Predicted Structural Revision", J. Am. Chem. Soc., 2012, 134, 18550–18553. {{DOI|10.1021/ja3089394}
- ↑ L. A. Paquette, N. A. Pegg, D. Toops, G. D. Maynard and R. D. Rogers, "[3.3] Sigmatropy within 1-vinyl-2-alkenyl-7,7-dimethyl-exo-norbornan-2-ols. The first atropselective oxyanionic Cope rearrangement", J. Am. Chem. Soc., 1990, 112, 277–283. DOI:10.1021/ja00157a043
- ↑ M. Ďurík, V. Langer, D. Gyepesová, J. Mičová, B. Steiner and M. Koóš, "1,2:4,5-Di-O-isopropylidene-β-d-erythro-hexo-2,3-diulo-2,6-pyranose", Acta Crystallogr. Sect. E, 2001, 57, 672–674. DOI:10.1107/S160053680101073X
- ↑ G. A. Jeffrey, J. A. Pople and L. Radom, "The application of ab initio molecular orbital theory to the anomeric effect. A comparison of theoretical predictions and experimental data on conformations and bond lengths in some pyranoses and methyl pyranosides", Carbohydr. Res., 1972, 25, 117–131.DOI:10.1016/S0008-6215(00)82752-4
- ↑ J. W. Yoon, T.-S. Yoon, S. W. Lee and W. Shin, "(1R,2R)-(–)-[Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine]chloromanganese(III), an (R,R)-Jacobsen catalyst", Acta Crystallogr. Sect. C, 1999, 55, 1766–1769. DOI:10.1107/S0108270199009397
- ↑ E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker and L. Deng, "Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane", J. Am. Chem. Soc., 1991, 113, 7063–7064. DOI:10.1021/ja00018a068
- ↑ F. R. Jensen and R. C. Kiskis,"Stereochemistry and mechanism of the photochemical and thermal insertion of oxygen into the carbon-cobalt bond of alkyl(pyridine)cobaloximes", J. Am. Chem. Soc., 1975, 97, 5825–5831. DOI:10.1021/ja00853a029
- ↑ H. Lin, J. Qiao, Y. Liu and Z.-L. Wu, "Styrene monooxygenase from Pseudomonas sp. LQ26 catalyzes the asymmetric epoxidation of both conjugated and unconjugated alkenes", J. Mol. Catal. B Enzym., 2010, 67, 236–241. DOI:10.1016/j.molcatb.2010.08.012
- ↑ 23.0 23.1 G. Fronza, C. Fuganti, P. Grasselli and A. Mele, "The mode of bakers' yeast transformation of 3-chloropropiophenone and related ketones. Synthesis of (2S)-[2-2H]propiophenone, (R)-fluoxetine, and (R)- and (S)-fenfluramine" J. Org. Chem., 1991, 56, 6019–6023. DOI:10.1021/jo00021a011 Cite error: Invalid
<ref>tag; name "RRTBMSOORP" defined multiple times with different content - ↑ E. Giorgio, R. G. Viglione, R. Zanasi and C. Rosini, "Ab Initio Calculation of Optical Rotatory Dispersion (ORD) Curves: A Simple and Reliable Approach to the Assignment of the Molecular Absolute Configuration", J. Am. Chem. Soc., 2004, 126, 12968–12976. DOI:10.1021/ja046875l
- ↑ H. Tian, X. She, J. Xu and Y. Shi, "Enantioselective Epoxidation of Terminal Olefins by Chiral Dioxirane", Org. Lett., 2001, 3, 1929–1931. DOI:10.1021/ol010066e
- ↑ J. M. Keith, J. F. Larrow and E. N. Jacobsen, Practical Considerations in Kinetic Resolution Reactions, Adv. Synth. Catal., 2001, 343, 5–26. DOI:10.1002/1615-4169(20010129)343:1%3C5::AID-ADSC5%3E3.0.CO;2-I
- ↑ S. Adelt, O. Plettenburg, R. Stricker, G. Reiser, H.-J. Altenbach and G. Vogel, "Enzyme-Assisted Total Synthesis of the Optical Antipodes d-myo-Inositol 3,4,5-Trisphosphate and d-myo-Inositol 1,5,6-Trisphosphate: Aspects of Their Structure−Activity Relationship to Biologically Active Inositol Phosphates", J. Med. Chem., 1999, 42, 1262–1273. DOI:10.1021/jm981113k




