Rep:MOD:JAP10537443
Title
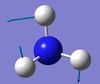
NH3 Molecule
N-H Bond Length = 1.02 Å
H-N-H Bond Angle = 106°
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -56.55776873 a.u.
RMS Gradient Norm = 0.00000485 a.u
Point Group = C3v
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy = -5.986283D-10
Ammonia Molecule |
A link to the .log file can be found here.
Below is a table displaying the vibrational frequencies and corresponding intensities that NH3 undergoes:
| Wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity a.u. | 145 | 14 | 14 | 1 | 0 | 0 |
| Image |  |
 |
 |
 |
 |
|
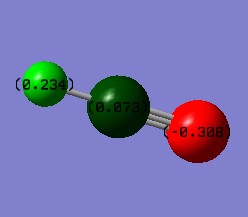
I expect the nitrogen atom to have a negative charge as nitrogen is more electronegative than hydrogen.
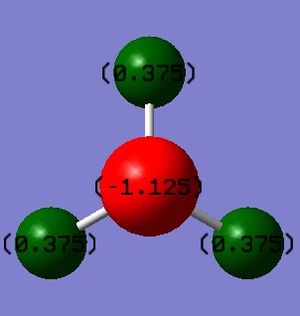
Furthermore, I expect the 3 hydrogen atoms to have positive charges of equal value as they are less electronegative than the nitrogen atom, and are all in the same environment. This is in fact true, and is highlighted by 'Figure 1 - NH3 Charge Distribution' on the right:
Below is more NH3 vibrational information:
Expected modes = 6
Degenerate modes = The two vibrations at 1694cm-1, and the two vibrations at 3590cm-1.
"Bending" vibrations = The vibration at 1090cm-1 and both the vibrations at 1694cm-1 are "bending" vibrations.
"Bond stretch" vibrations = The vibration at 3461cm-1 and both the vibrations at 3590cm-1 are "bond stretch" vibrations.
Highly symmetric mode = The vibration at 3461cm-1 is highly symmetric.
"Umbrella" mode = The mode at 1090cm-1 is known as the "umbrella" mode.
Number of bands in an experimental spectrum of gaseous ammonia = 3
N2 Molecule
N-N Bond Length = 1.11 Å
N-N Bond Angle = 180°
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -109.52412868 a.u.
RMS Gradient Norm = 0.00001838 a.u.
Point Group = D*H
Item Value Threshold Converged? Maximum Force 0.000032 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000010 0.001800 YES RMS Displacement 0.000014 0.001200 YES Predicted change in Energy=-3.168264D-10
Nitrogen Molecule |
A link to the .log file can be found here.
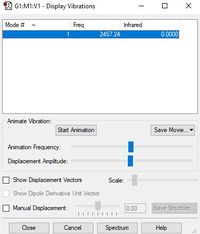
Below is a table displaying the vibrational frequencies and corresponding intensities that N2 undergoes:
| Wavenumber cm-1 | 2457 |
| Symmetry | SGG |
| Intensity a.u. | 0 |
| Image | 
|
Linked [| here] is a mono-metallic transition metal (TM) complex that coordinates N2 - Dinitrogen-pentakis(trimethylphosphine)-molybdenum (BIWGAP10).
The bond distance of N2 is 1.11Å. The bond distance between the two nitrogen atoms in this mono-metallic TM complex is slightly greater at 1.12Å [1] . They are very similar because the two nitrogen atoms are, like in N2, triple bonded to each other. However, in the mono-metallic TM complex, some of the electron density is drawn into the Mo-N bond, reducing the electron density in the triple bond between the two nitrogen atoms and so slightly increasing the bond length. Further more, the N triple-bond N bond length of the diatomic, N2, was determined computationally, which uses approximations to predict the bond distance. This is unlike the bond distance determined in the mono-metallic TM complex which was determined experimentally. This could also account for the 0.01Å difference.
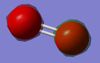
I expect there to be no charge on either N2 atom because both atoms in the diatomic are the same and in the same environment and hence, have the same electronegativity. This is in fact true and is highlighted by 'Figure 2 - N2 Charge Distribution' on the right:
H2 Molecule
H-H Bond Length = 0.74 Å
H-H Bond Angle = 180°
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -1.17853929 a.u.
RMS Gradient Norm = 0.0001307 a.u.
Point Group = D*H
Item Value Threshold Converged? Maximum Force 0.000226 0.000450 YES RMS Force 0.000226 0.000300 YES Maximum Displacement 0.000298 0.001800 YES RMS Displacement 0.000422 0.001200 YES Predicted change in Energy=-6.751273D-08
Hydrogen Molecule |
A link to the .log file can be found here.
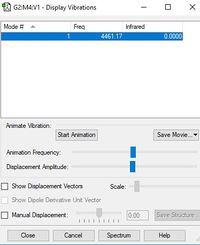
Below is a table displaying the vibrational frequencies and corresponding intensities that H2 undergoes:
| Wavenumber cm-1 | 4461 |
| Symmetry | SGG |
| Intensity a.u. | 0 |
| Image | 
|
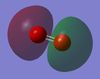
I expect there to be no charge on either H2 atom because both atoms in the diatomic are the same and in the same environment. Hence, they have the same electronegativity. This is in fact true as shown by 'Figure 3 - H2 Charge Distribution' on the right:
Haber-Bosch reaction energy calculation
Determining the energy for the Haber-Bosch reaction (N2(g) + 3H2(g) → 2NH3(g)):
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853929 a.u.
3*E(H2)= -3.53561787 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -113.11553746 - [-109.52412868 + (-3.53561787)] a.u.
ΔE= -0.05579091 a.u. = -0.05579091 * 2625.5 KJ/mol = -146.479034205 KJ/mol
ΔE= -146.5 KJ/mol (1dp)
The ammonia product is more stable than the gaseous reactants as this is an exothermic reaction and the ammonia is at a lower energy.
Molecule of my own choice - O2
O-O Bond Length = 1.22 Å
O-O Bond Angle = 180°
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -150.25742435 a.u.
RMS Gradient Norm = 0.00001851 a.u.
Point Group = D*H
Item Value Threshold Converged? Maximum Force 0.000032 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000020 0.001800 YES RMS Displacement 0.000028 0.001200 YES Predicted change in Energy=-6.129527D-10
Oxygen Molecule |
A link to the .log file can be found here.
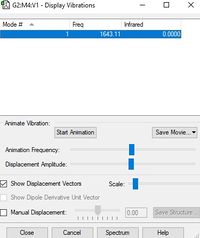
Below is a table displaying the vibrational frequencies and corresponding intensities that O2 undergoes:

| Wavenumber cm-1 | 1643 |
| Symmetry | SGG |
| Intensity a.u. | 0 |
| Image | 
|
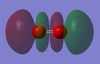
I expect there to be no charge on either O2 atom because both atoms in the diatomic are the same and in the same environment and hence, have the same electronegativity. This is in fact true as shown by 'Figure 4 - O2 Charge Distribution' on the right:
Note - the LUMO shown on Gaussian is actually the π*2py MO because the calculated energies of the π*2px and π*2py MOs aren't exactly the same. As a result of a restricted optimsation being used and Gaussian strictly following Hund's rule of maximum multiplicity, 2 electrons occupy the π*2px, resulting in the π*2py being unoccupied. In real life, the two degenerate π*2px and π*2py orbitals are SOMOs (both contain one electron) and the LUMO is the orbital highlighted in the table above - the σ*2pz orbital.
Extra Molecule - HCN
H-C Bond Length = 1.01 Å
C-N Bond Length = 1.16 Å
H-C-N Bond Angle = 180°
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -93.42458152 a.u.
RMS Gradient Norm = 0.00002060 a.u.
Point Group = C*V
Item Value Threshold Converged? Maximum Force 0.000047 0.000450 YES RMS Force 0.000031 0.000300 YES Maximum Displacement 0.000078 0.001800 YES RMS Displacement 0.000049 0.001200 YES Predicted change in Energy=-2.816950D-09
Hydrogen Cyanide Molecule |
A link to the .log file can be found here.
Below is a table displaying the vibrational frequencies and corresponding intensities that HCN undergoes:
| Wavenumber cm-1 | 770 | 770 | 2213 | 3475 |
| Symmetry | PI | PI | SG | SG |
| Intensity a.u. | 35 | 35 | 2 | 57 |
| Image |  |
 |
 |
|
I expect there to be a negative charge on the nitrogen atom as this is a fairly electronegative atom. However, hydrogen and carbon are not very electronegative, hence I expect them to have positive values. This is in fact correct and is highlighted by 'Figure 5 - HCN Charge Distribution' on the right:
References
- ↑ Galindo, A.; Gutiérrez, E.; Monge, A.; Paneque, M.; Pastor, A.; Pérez, P.; Rogers, R.; Carmona, E. J. Chem. Soc., Dalton Trans. 1995, 3801-3808.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES, but it helps the reader to use the built in subheadings from which the wiki can automatically generate a table of contents.
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES, most answers are correct. However there are only 2 visible peaks in the spectra of NH3, due to the low intensity of the other 2 peaks. (See infrared column in vibrations table.)
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES, However you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES, good exaplanation well done!
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES, very good explanation about the restricted calculation, well done!
Independence 1/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
Do an extra calculation on another small molecule, or
You calculated and analysed HCN well done!
Do some deeper analysis on your results so far