Rep:MOD:CID01260105
NH3
Molecular Information
| Category | Information |
|---|---|
| N - H Bond Length | 1.01798Å |
| H-N-H Bond Angle | 105.741° |
| Calculation Method | R3BLYP |
| Basis Set | 6-31(d,p) |
| E(R3BLYP) | -56.55776873 a.u. |
| RMS Gradient Norm | 0.00000485 a.u. |
| Point Group | D∞h |
| Calculation Log | NH3 Optimisation |
NH3 Molecule |
Convergence Confirmation
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986292D-10
Optimization completed.
-- Stationary point found.
Vibrational Data
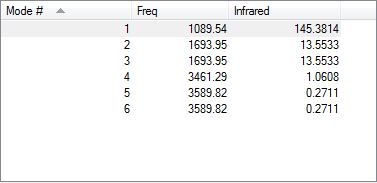
i) Given that there are 4 atoms in this non-linear molecule, N=4, 3N-6 = 12-6 =6. Thus 6 vibrational modes were expected and found
ii) There are two pairs of degenerate bond vibrations listed at Mode # 2-3 and 5-6
iii) The bending vibrations are Modes: 1, 2 and 3. The stretching vibrations are 4, 5 and 6.
iv) Mode #4, (Frequency:3461.29) is the most highly symmetric vibration
v) Mode #1 (Frequency:1089.54) is the "umbrella" mode due to its resemblance of an umbrella being pushed inside out
vi) Two IR absorption bands are expected. Mode # 4, 5 & 6 have very low intensities (1.0608, 0.2711 and 0.2711 respectively); these will only produce negligible peaks on an IR spectrum. As mentioned, Mode # 2 & 3 are degenerate, producing a single band. This results it 2 absorption bands.
Charge Analysis

With the central atom in NH3 being the most electronegative, it is expected that the N atom would have the most negative charge/highest electron density. Given that there are three identical monoatomic substituents (three H atoms, all in the same environment), each of these will have the same electron density. Additionally, given the neutral nature of the molecule, the sum of the partial charges on the four atoms must equal -1.125 +3(0.375) = 0
H2
Molecular Information
| Category | Information |
|---|---|
| H - H Bond Length | 0.74279Å |
| Calculation Method | R3BLYP |
| Basis Set | 6-31(d,p) |
| E(R3BLYP) | -1.17853936 a.u. |
| RMS Gradient Norm | 0.00000017 a.u. |
| Point Group | D∞h |
| Calculation Log | H2 Optimisation |
H2 Molecule |
Convergence Confirmation
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
Vibrational Data
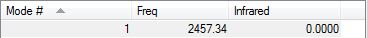
A 0 value occurs for the IR intensity due to the lack of change in dipole moment (given the diatomic molecule)
N2
Molecular Information
N ≡ N Bond Length = 1.10550Å
Calculation Method: R3BLYP
Basis Set: 6-31G(d.p)
E(R3BLYP) = -109.52412868 a.u.
RMS Gradient Norm = 0.00000217 a.u.
Point Group: D∞h
Calculation Log: Media:JJ1516_N2_OPTIMISED.LOG
N2 Molecule |
Convergence Confirmation
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000001 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-4.428717D-12
Optimization completed.
-- Stationary point found.
Vibrational Data
As with the diatomic H2 molecule, there is no change in the dipole moment due to the N - N bond vibration; the molecule is IR inactive, hence the 0 intensity value.
Reaction Energies
Haber-Bosch Process
N2(g) + 3H2(g) → 2NH3
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -113.11553746 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853936 a.u.
3*(H2) = -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u. = -146.48 kJ•mol-1
ΔE(literature value) = -45.94 ± 0.35 kJ•mol-1 [1]
ΣE(reactants) = -113.05974676 a.u.
ΣE(products) = -113.11553746 a.u.
The value and (negative) sign of ΔE indicate that the products (2 molecules of NH3) are more thermodynamically stable than the reactants (3 molecules of H2 and one molecule of N2). Therefore, the Haber-Bosch Process is highly exothermic.
The large discrepancy between the calculated and experimental ΔE values arises chiefly due to the method of energy calculation employed. The computational procedure used by GaussView relies primarily upon Density Functional Theory; the approximations invoked by DFT when dealing with quantum mechanical electron interactions (e.g. exchange and correlation interactions) cause the computed data to differ from real-world, experimental results.
F2
Molecular Information
| Category | Information |
|---|---|
| F - F Bond Length | 1.40317Å |
| Calculation Method | R3BLYP |
| Basis Set | 6-31(d,p) |
| E(R3BLYP) | -199.49825217 a.u. |
| RMS Gradient Norm | 0.00008442 a.u. |
| Point Group | D∞h |
| Calculation Log | F2 Optimisation |
F2 Molecule |
Convergence Information
Item Value Threshold Converged?
Maximum Force 0.000146 0.000450 YES
RMS Force 0.000146 0.000300 YES
Maximum Displacement 0.000180 0.001800 YES
RMS Displacement 0.000254 0.001200 YES
Predicted change in Energy=-2.627106D-08
Optimization completed.
-- Stationary point found.
Vibrational Data
As with the diatomic N2 molecule, there is no change in the dipole moment due to the F - F bond vibration; the molecule is IR inactive, hence the 0 intensity value.
Charge Analysis
Due to the diatomic nature of the molecule, there is no electronegativity difference between the two atoms.
Thus, F2 is a perfectly covalent molecule, thus the partial charges on the two atoms is 0.

Molecular Orbital Analysis
References
- ↑ Cox, Wagman, et al., 1984 Cox, J.D.; Wagman, D.D.; Medvedev, V.A., CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, 1984, 1