Rep:MOD:01353582
N2
Basic Information
| Formula | Calculation Method | Basis Set | E(RB3LYP) | RMS Gradient | Point Group | Bond Distances | Bond Angles | |
|---|---|---|---|---|---|---|---|---|
| Calculated Value | N2 | RB3LYP | 6-31G(d,p) | -109.52359111 au | 0.00000060 au | D∞S | 1.1055 Å | N/A |
| Literature Value[1] | 1.098 Å |
Items Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Optimisation file available here: File:BS517 N2 OPTF POP.LOG
Model
Nitrogen |
Vibrations
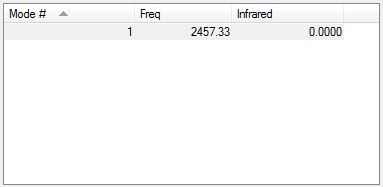
No IR active vibrations available.
H2
Basic Information
| Formula | Calculation Method | Basis Set | E(RB3LYP) | RMS Gradient | Point Group | Bond Distances | Bond Angles | |
|---|---|---|---|---|---|---|---|---|
| Calculated Value | H2 | RB3LYP | 6-31G(d,p) | -1.17853936 au | 0.00000017 au | D∞S | 0.7428 Å | N/A |
| Literature Value[1] | 0.741 Å |
Items Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Optimisation file available here: File:BS517 H2 OPTF POP.LOG
Model
Hydrogen |
Vibrations
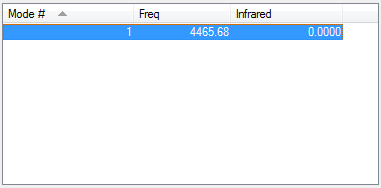
No IR active vibrations available.
NH3
Basic Information
| Formula | Calculation Method | Basis Set | E(RB3LYP) | RMS Gradient | Point Group | Bond Distances | Bond Angles | |
|---|---|---|---|---|---|---|---|---|
| Calculated Value | NH3 | RB3LYP | 6-31G(d,p) | -56.55776873 au | 0.00000485 au | C3V | 1.01798 Å | 105.741° |
| Literature Value[1] | 1.012 Å | 106.67° |
Model
Ammonia |
Items Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Optimisation file available here: File:BS517 NH3 OPTF POP.LOG
Vibrations
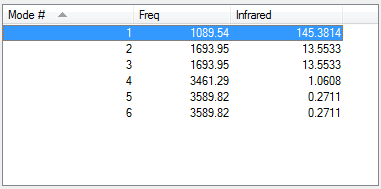
From the 3N-6 rule 6 vibrational modes are expected, 1-3 are bending modes whereas 4-6 are streaching modes. 1 and 4 are highly symmertic, 1 is called the umbrella mode. There are two pairs of degenerate modes, (2, 3) and (5, 6). Although the change in dipole moment is not zero for either of the modes, because of their relative values only two peaks will be present on experimental spectra.
Charge Distribution
The N atom is expected to have an electric charge of -1.125 while the H-s have a charge of 0.375 each.
Reactivity
Synthesis by the Haber-Bosch Process
N2 + 3H2 -> 2NH3
Reaction Energy Calculation
E(NH3) = -56.55776873 au 2*E(NH3) = -113.11553746 au E(N2) = -109.52359111 au E(H2) = -1.17853936 au 3*E(H2) = -3.53561808 au ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = -0.056328269 au = -147.88988415 kJ/mol Reaction is exothermic, product is more stable than reactants.
CH4
Basic Information
| Formula | Calculation Method | Basis Set | E(RB3LYP) | RMS Gradient | Point Group | Bond Distances | Bond Angles | |
|---|---|---|---|---|---|---|---|---|
| Calculated Value | CH4 | RB3LYP | 6-31G(d,p) | -40.52401404 au | 0.00003263 au | D∞S | 1.09197 Å | 109.47122° |
| Literature Value[1] | 1.087 Å | 109.471° |
Model
Methane |
Items Table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES
Optimisation file available here: File:BS517 CH4 OPTF POP.LOG
Vibrations
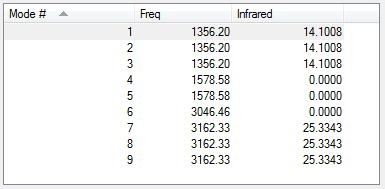
From the 3N-6 rule 9 vibrational modes are expected, 1-5 are bending modes whereas 6-9 are streaching modes. 5 and 6 are symmetric. There are multiple degenerate modes, (1, 2, 3) and (4, 5) and (7, 8, 9). We see two peaks in experimental IR spectra of methane since the symmetric modes don't have a change in dipole moment associated with them, one for the bending and one for the streaching modes.
Charge Distribution
The C atom is expected to have an electric charge of -0.93 while the H-s have a charge of 0.233 each.
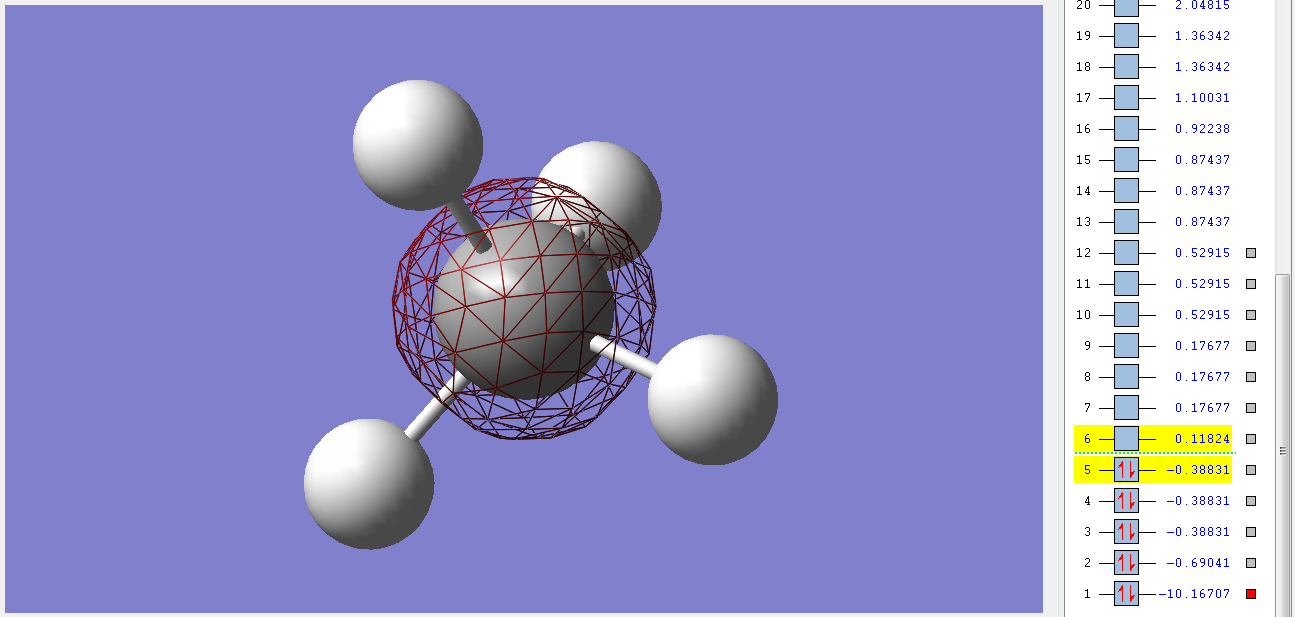
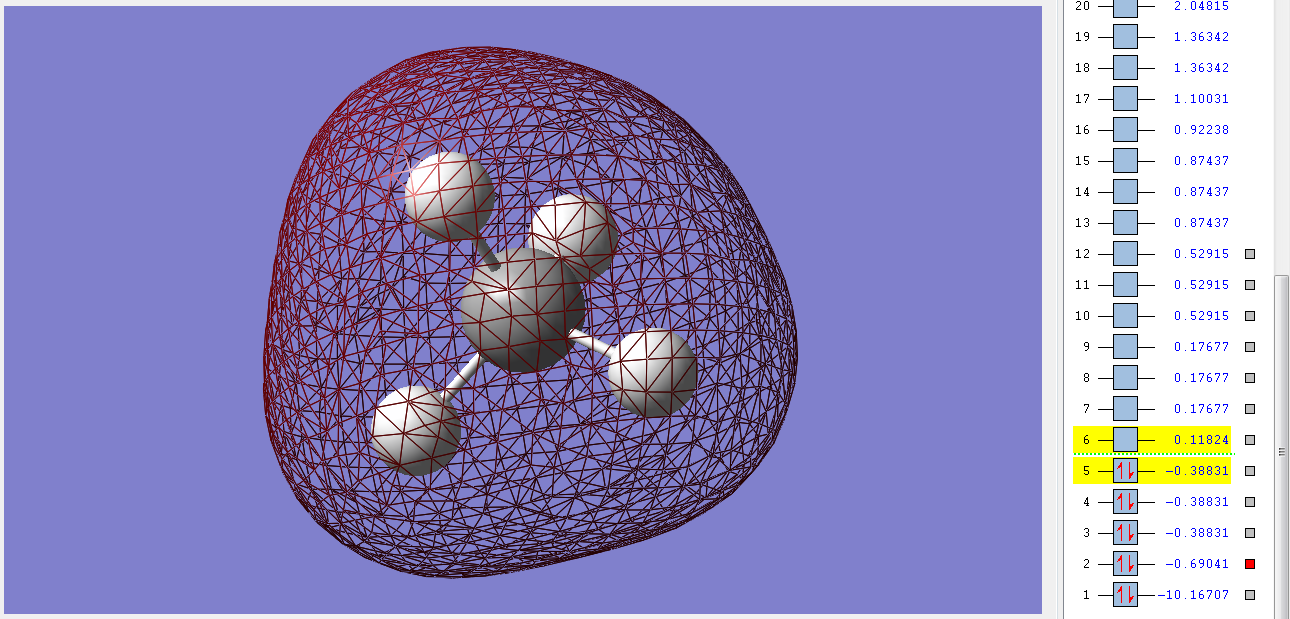
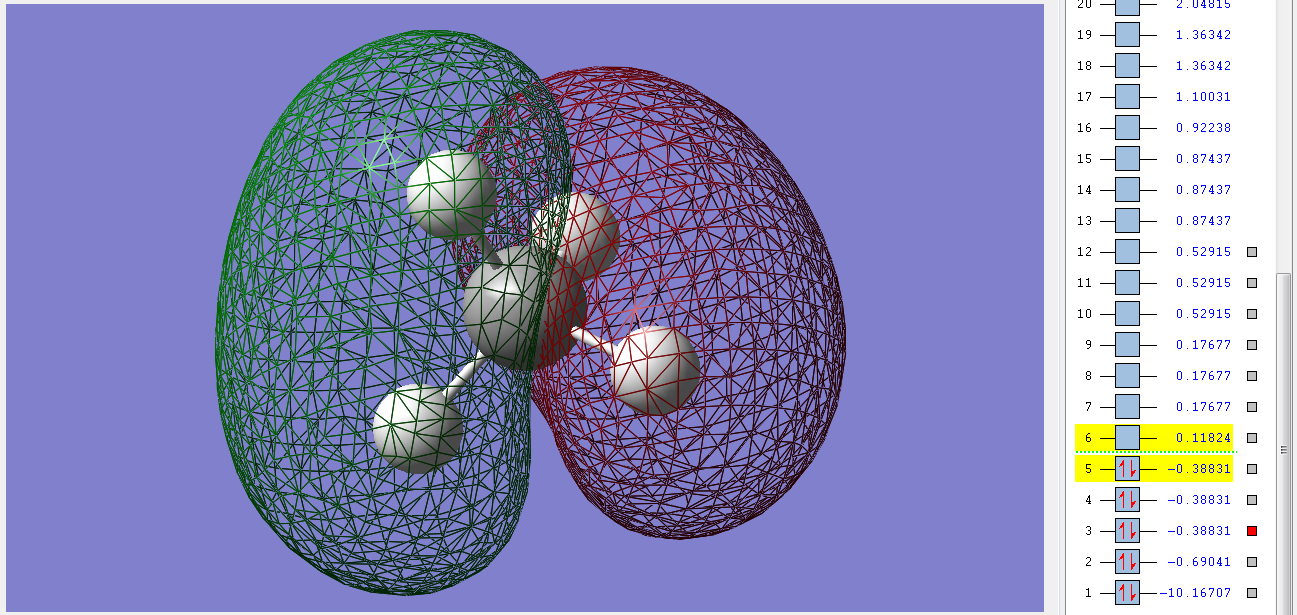
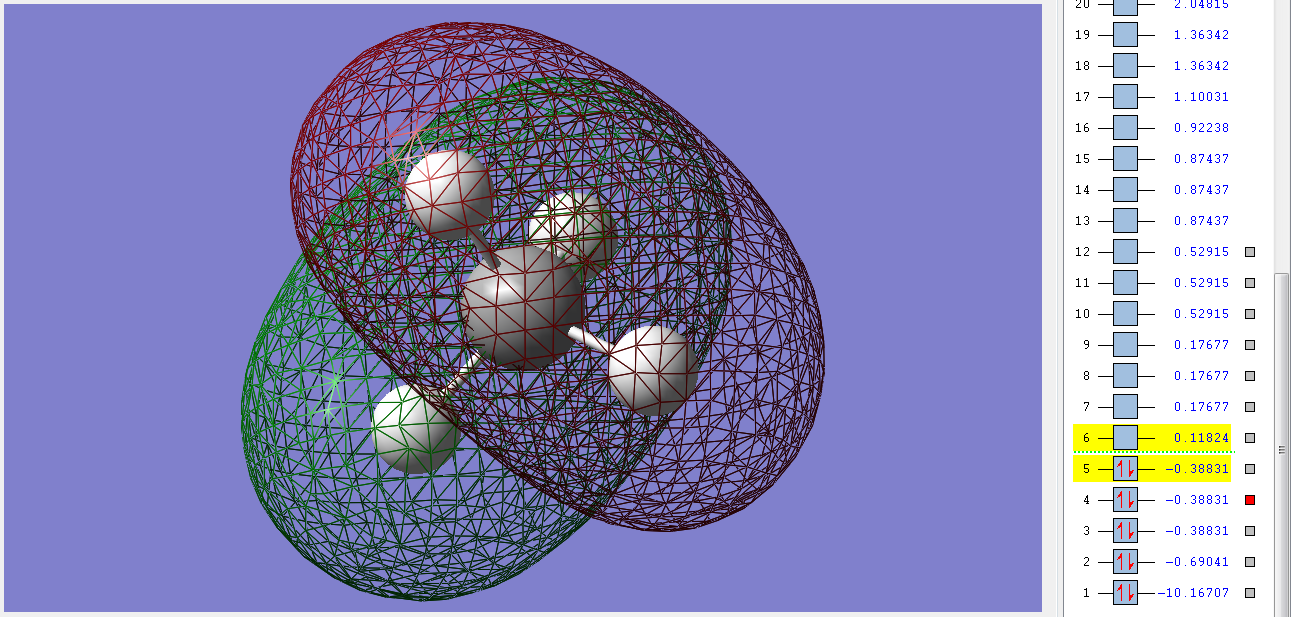
Molecular Orbitals
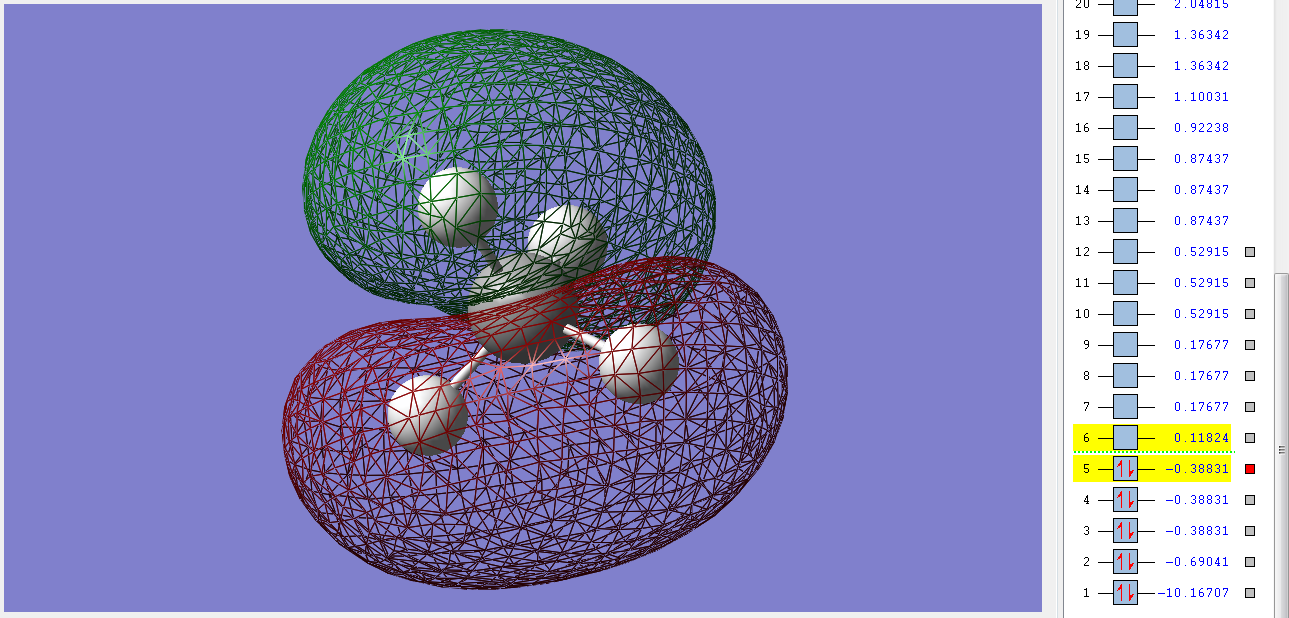
1sg non-bonding orbital
This orbital is the deepest in energy. It's made up of the 1s orbital of the C atom, which is too deep in energy for the electrons of the H atoms to interact with it in any significant manner. It's concentrated very close to the nucleus of the C and so we call it a non-bonding orbital. It's filled with 2 electrons and has a gerade symmetry.
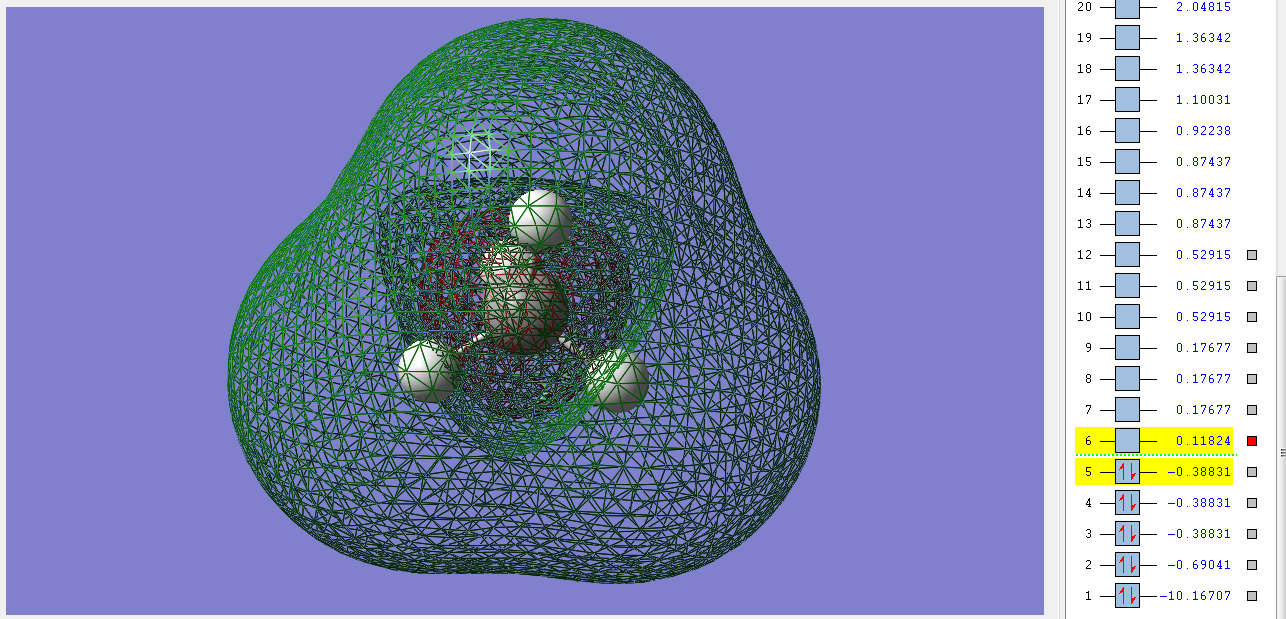
1σg
This is the first bonding orbital of the methane molecule. It's made from the combination of the 2s orbital of the C and the 1s orbitals of the Hs. It's filled with two electrons is very deep in energy and has a gerade symmetry. This orbital is still deep in energy because of the sp mixing of the orbitals of the C. There is a higher electron density on the Hs from this orbital.
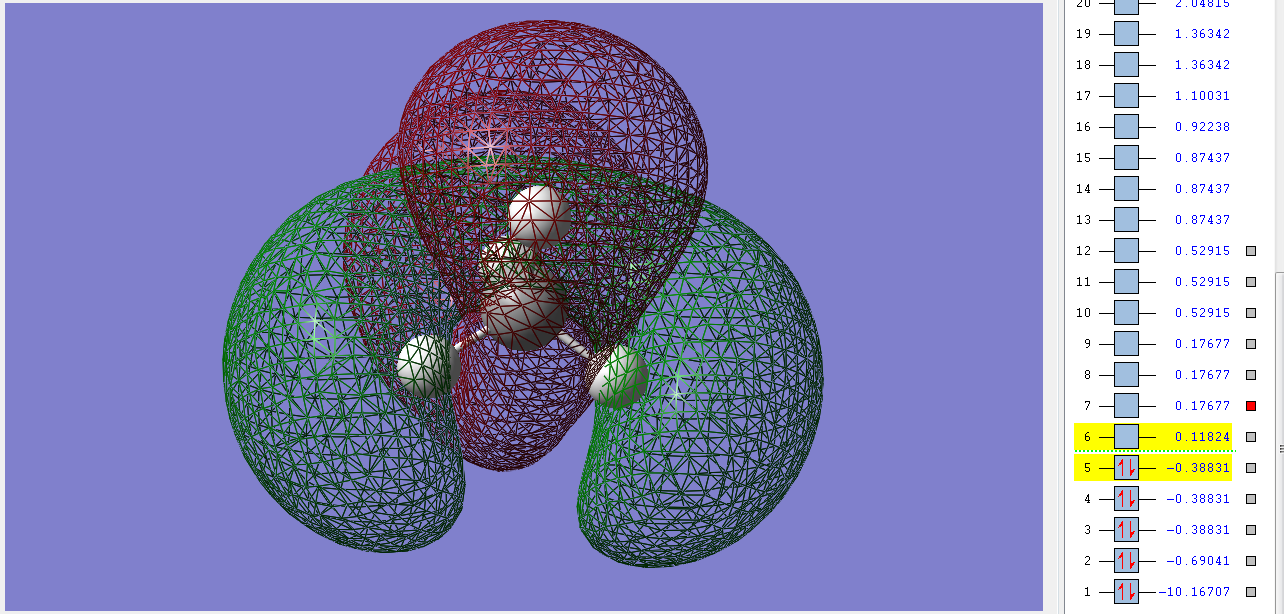
2σu
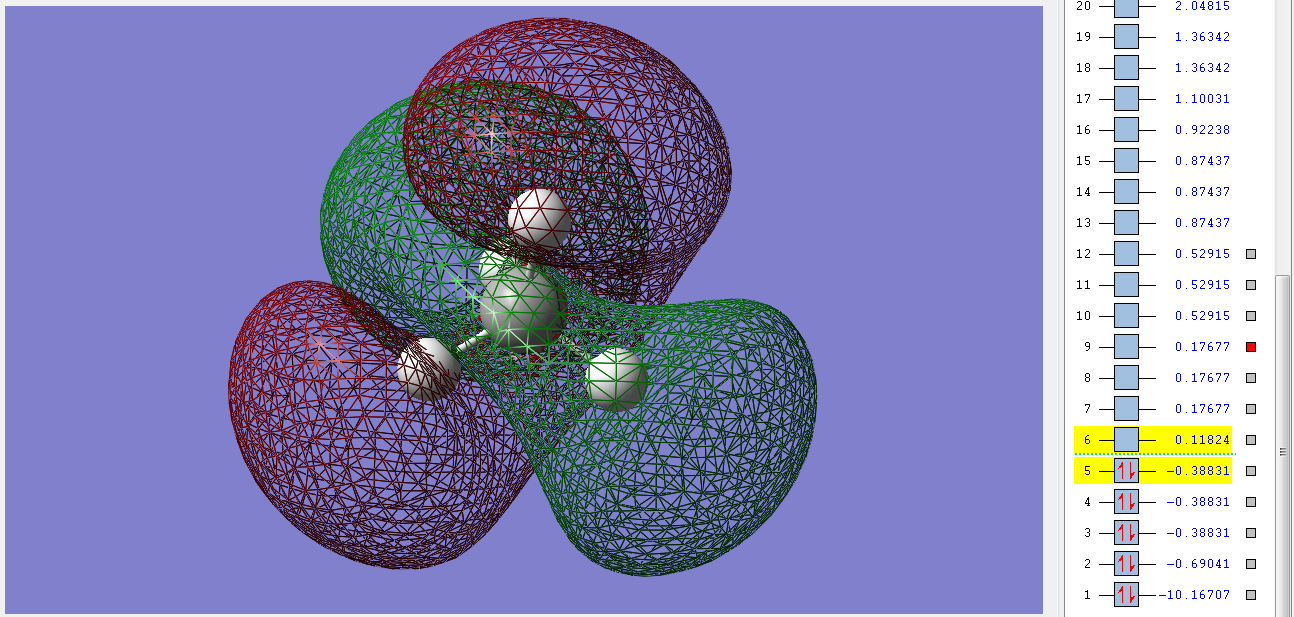
These 3 degenerate bonding orbitals are the HOMOs of methane. They are filled with two electrons each and have an ungerade symmerty. They are made up of the combination of the 2p orbitals of the C and the 1s orbitals of the Hs and the electrons lie closer to the Hs. The stability of methane in part comes from the fact that it has no filled antibonding orbitals, all of those are unfilled.
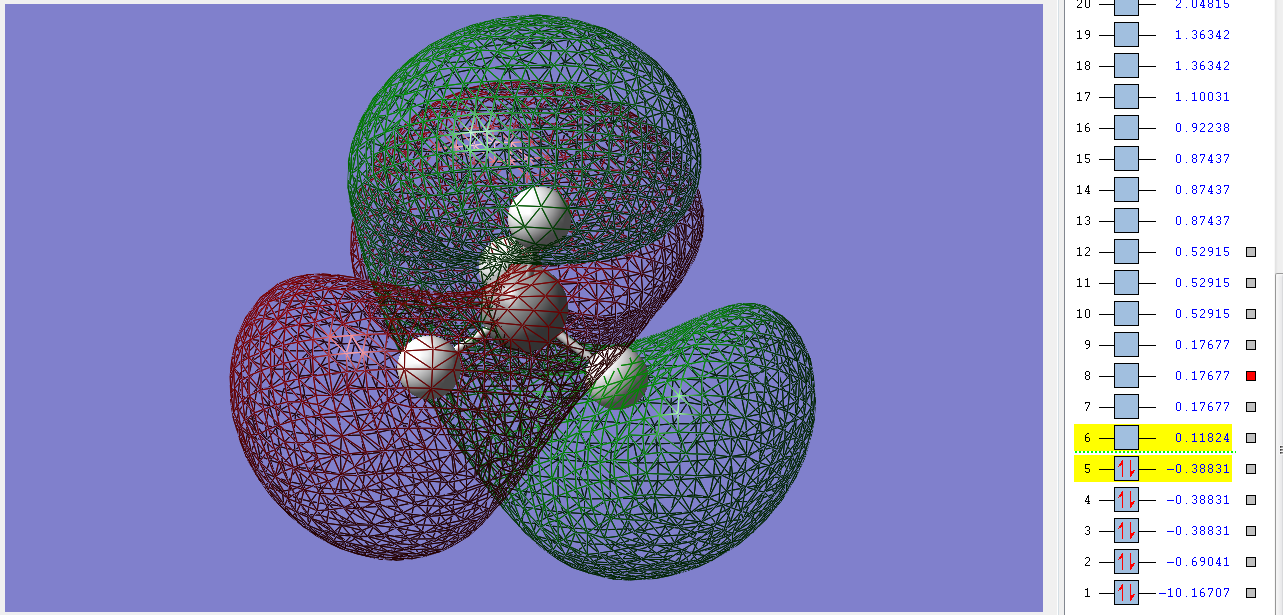
3σ*g
This unfilled antibonding orbital is the LUMO of methane. It's very symmetryc (gerade) and is made up of the combination of the 1s orbitals of the Hs and the 2s orbital of the carbon. It's pushed up to a higher energy then expected due to the sp mixing of the orbitals of the C.
4σ*u
These three degenerate unfilled antibonding orbitals are high in energy, but not much higher than the LUMO. They have an ungerade symmetry and are pushed up in energy due to the sp mixing of the orbitals of the C. They are made up of the combination of the Hs' 1s orbitals and the 2p orbitals of the C.
References
Literature values:
- ↑ 1.0 1.1 1.2 1.3 Computational Chemistry Comparison and Benchmark DataBase Release 18 (October 2016),Standard Reference Database 101,National Institute of Standards and Technology, https://cccbdb.nist.gov/